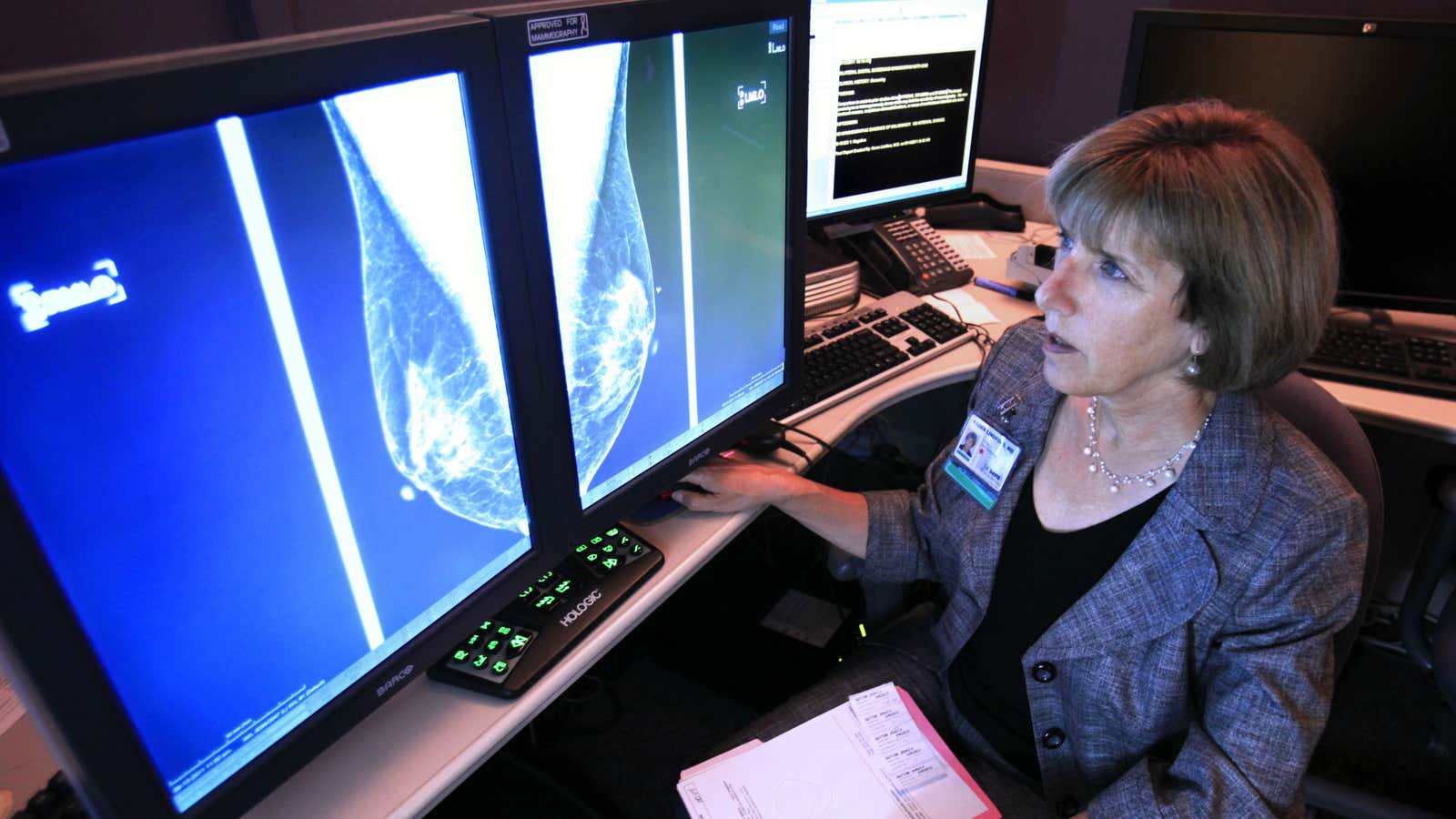
The best way to screen for breast cancer is a mammogram, but some companies have been trying to convince American women otherwise.
Last week, the US Food and Drug Administration issued a warning about advertisements for thermograms falsely claiming the technology could be as good or better than mammograms. In 1982, the FDA approved thermograms for breast-cancer screening—but only in conjunction with regular mammograms. Although thermography is not harmful in and of itself, there’s no scientific evidence that actually can detect breast cancer on its own.
Since 2016, the FDA has issued two warnings to companies advertising preventative breast cancer screenings. Nature’s Treasures claimed earlier this year that “thermography is far more sensitive than mammography,” and that “thermography can detect the possibility of breast cancer much earlier.” The company website is now “under maintenance.” The other company in question, Thermogram Assessment Services, was reportedly using equipment that had not been approved by the FDA to perform thermograms. This company still appears to be operational, and they haven’t posted a response yet.
Thermograms are basically just an image of the breasts taken with a thermal camera. They look for additional heat and blood-vessel formation that can theoretically show where cancer may be growing. The main advantage of breast thermography is that it’s a lot less invasive than a mammogram, and there’s no radiation involved. (Radiation can cause cancer itself, but the benefit of breast-cancer detection with routine screenings greatly outweighs these risks.) In addition, mammograms can be uncomfortable because they often involve squeezing the breast tissue between two plates to get better contrast between noncancerous and any cancerous cells.
Although rates of breast cancer detection has gone up in recent years, deaths from breast cancer have decreased by 39% since 1989. According to the American Cancer Society (pdf, p. 9), this is because detection through mammograms has caught more breast tumors earlier. The US Preventative Task Force recommends that all women aged 50 to 74 should have a mammogram every two years. Women who are at a higher risk for breast cancer through a family history or known genetic risks should start these screenings sooner.
“The greatest danger from thermography is that those who opt for this method instead of mammography may miss the chance to detect cancer at its earliest stage,” the FDA writes. The regulatory agency is on the lookout for other companies falsely advertising the cancer-detecting abilities of thermograms.