Immunotherapy can be an effective treatment against cancer. But over the past 30 years, research into immunotherapy has nearly been abandoned several times. And if no one had ever picked it back up, it might have been bad news for former US president Jimmy Carter.

Last August, Carter announced he had stage IV metastatic melanoma, and that it had spread to his brain. When he first got the diagnosis, he thought he might have only “a few weeks left,” he said, but by the time of his press conference, he was more optimistic. Carter would soon undergo surgery and radiation treatment, and would also receive regular doses of pembrolizumab, an immunotherapy drug that had been approved by the Food and Drug Administration (FDA) to treat melanoma less than a year earlier.
And four days ago, after about three and a half months of treatment, Carter announced that his cancer was gone.
The former president, who is now 91, said in a statement: “My most recent MRI brain scan did not reveal any signs of the original cancer spots nor any new ones. I will continue to receive regular three-week immunotherapy treatments of pembrolizumab.”
Friend or foe
The body’s immune system doesn’t normally attack cancer. This is because cancer cells are just the body’s own cells, mutated. Immunotherapy works by tweaking the immune system to unmask cancerous cells as antigens—that is, as enemies.
Different immunotherapies do this in different ways, but pembrolizumab achieves it by blocking protein receptors on certain kinds of white blood cells (specifically, B- and T-cells). These receptors act as the immune system’s “brake”; they’re a safety mechanism, essentially, to prevent the white blood cells attacking healthy tissue. By blocking them, pembrolizumab releases that brake. It means some healthy cells will be destroyed along with malignant ones, but the benefit usually outweighs the cost.
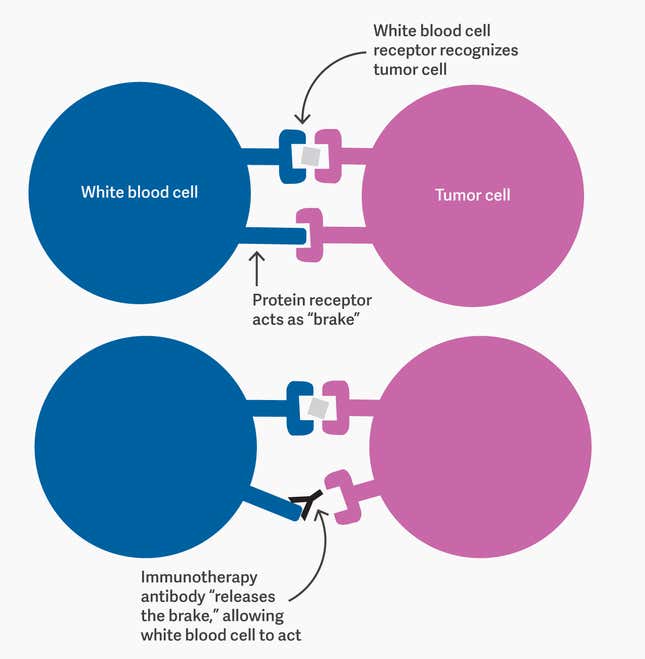
Developing this technique took a series of breakthroughs over more than a century of research, as well as persistence in the face of multiple failures. It all started in Berlin with a physiologist, a mouse, and a guinea pig.
In the beginning
In 1890, Emil von Behring, a German physiologist, was looking for a new way to fight infections. He had a guinea pig that had just recovered from diphtheria, an infectious disease caused by bacteria. He also had a healthy mouse. He infected the mouse with diphtheria, then treated it with blood from the recovered guinea pig, and the mouse was cured; in fact, it was now immune to diphtheria. For that discovery, Behring won a Nobel prize in 1901.
Behring and a contemporary of his, the German physician Paul Ehrlich, laid the foundations for our understanding of how antibodies work. Antibodies are Y-shaped proteins that white blood cells release when they’re under threat. They serve a bit like jamming devices: They block the protein receptors on harmful antigens, neutralizing them. They can also serve as warning markers: When an antigen is covered in antibodies, it’s flagged as a target for another part of the immune system to attack. Behring’s mouse recovered from diphtheria because the blood it got from the guinea pig already contained diphtheria antibodies, making it easier for the mouse’s immune system to identify and attack the disease.
Pembrolizumab, the immunotherapy used to treat Carter, is an antibody too. It’s just used in a different way. It doesn’t attack cancer cells, as diphtheria antibodies attack diphtheria. Instead, it acts on the white blood cells to release their “brakes,” so they can target cancer cells.
Pembrolizumab is also a specific type of antibody: a “monoclonal” antibody, or mAb, meaning one that has been synthesized in quantity starting from a small initial batch. Monoclonal antibodies are useful because they can bind with a specific substance, such as a particular cell receptor. The first mAbs were produced by injecting a mouse with an antigen (something its immune system would interpret as a danger); waiting for it to produce antibodies; extracting and isolating those; fusing them with constantly-replicating cells so they’d multiply; and finally, injecting the result into humans.
In the 1980s, there was a lot of excitement around using mAbs to treat cancer. In trials on mice, they reduced tumors time and time again. But the party didn’t last long. In clinical trials on humans, most patients got sicker than they were to begin with.
The reason was that the antibodies themselves had come from mice. The human immune system saw them as foreign, and reacted badly. At that point, many scientists abandoned immunotherapy research.
Ups and downs
The failures of mAbs weren’t the only reason immunotherapy fell into disfavor. Another kind of immunotherapy treatment, a drug called interferon, had had similar problems.
Interferon is made of proteins that white blood cells release in response to certain bacteria and viruses. The hope was that it would attack cancer cells the way those proteins attacked infections. It was the most promising new cancer treatment of the 1970s. Like mAbs, the drug successfully reduced tumors in mice. But, also like mAbs, it produced poor results in human trials.
So when an American medical researcher named James Allison began pursuing immunotherapy in the late ‘70s and early ‘80s, his peers discouraged him, suggesting he should avoid the embarrassment. But Allison was undeterred by the previous failures. He believed he could find a way to manipulate T-cells—a type of white blood cell— into recognizing cancer as a threat and start attacking it.
In the late 1980s Greg Winter, a biochemist at the UK’s Medical Research Council, made a crucial step forward. He figured out how to “humanize” antibodies taken from rats—essentially splicing them with bits of human antibody to make them look less foreign to the human body’s immune system.
By the mid-1990s, Allison had made progress as well. He and his team at the University of California at Berkeley had identified a molecule, CTLA-4, which they believed was the protein receptor that controlled the “brakes” on T-cells. So they developed a monoclonal antibody that could block that protein, and prompt T-cells to act.
The treatment they developed worked on mice, but when it came to human clinical trials—two separate ones, one funded by Bristol Myers-Squibb, the other by Pfizer—the results were, yet again, discouraging. For the most part, the patients’ tumors only grew bigger. The Pfizer trial was abandoned early. Hope seemed lost.
Several months later, however, the researchers in the Bristol Myers-Squibb trial—which was already over—noticed that their patients had started getting better. Their tumors had stopped growing and, in some cases, began to shrink. Years later, when the final reckoning was in, many of them had lived a good deal longer than they’d been expected to.
Allison’s immunotherapy drug is now called ipilimumab. It was approved by the FDA in 2011 under the Bristol Myers-Squibb brand name Yervoy, and it was the first “immune checkpoint blockade”—essentially a predecessor to pembrolizumab, the antibody that would be used to treat Jimmy Carter.
The success led to new optimism around immunotherapy. The journal Nature published a review of ipilimumab in 2011, titled “Cancer immunotherapy comes of age.” In 2013, largely citing Allison’s work, Science called immunotherapy the “breakthrough of the year.”
“It’s like a tree”
In the 2013 clinical trial that would lead the FDA to fast-track the approval of pembrolizumab (under the brand name Keytruda, marketed by Merck), only about a quarter of patients taking the standard dose of the drug fared as well as Carter would. Those may not sound like excellent odds, but the patients who did respond well continued to see improvement for several months after treatment stopped.
In Carter’s case, pembrolizumab was used along with radiation—a combination that the Cancer Research Institute says “may empower the immune system to mount an even more robust and effective attack on the cancer hiding in the body.”
In May 2013, I spoke with James Allison while researching an essay about my own experience with monoclonal antibodies. (In my case, the mAb was called rituximab, and the affliction was advanced Hodgkin’s lymphoma.) I asked him about the history of immunotherapy, some of which I also covered in my previous piece. I wanted to know why promising research can be so readily abandoned—why a few failures nearly led us to pass up this approach to cancer treatment.
“That’s the nature of science,” he said. “It’s not a linear progression; it’s like a tree, and most limbs snap off.”