“Likes repel; opposites attract.” Every school student is familiar with this rule, known as Coulomb’s law. If you bring two north ends of magnets or two negatively charged bodies close to each other, there is no escaping the force of repulsion. The closer you bring them, the greater the force you feel.
Now researchers at Indiana University in the US have found a way to defy the 200-year-old law. In doing so, they open up new applications that we hadn’t thought possible before: from easily treating nuclear waste to preventing algal blooms.
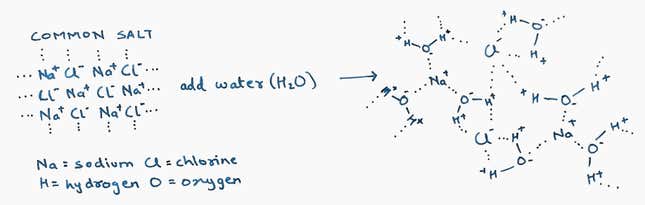
Many molecules are held together by attraction between opposite electrical charges. Common salt, for instance, is made up of equal numbers of sodium and chlorine atoms. They bond together as salt because the sodium atoms can acquire a positive charge and the chlorine atoms can acquire a negative one, so they attract each other. Atoms with a charge are called ions. When you add salt to water, the sodium and chlorine ions break their bonds with each other and starting forming new bonds with charges in water molecules—which is how salt dissolves.
These interactions between electrical charges govern a lot of chemistry. Amar Flood, professor of chemistry at Indiana University, has been studying them for many years. He’s been working with star-shaped molecules called cyanostars.
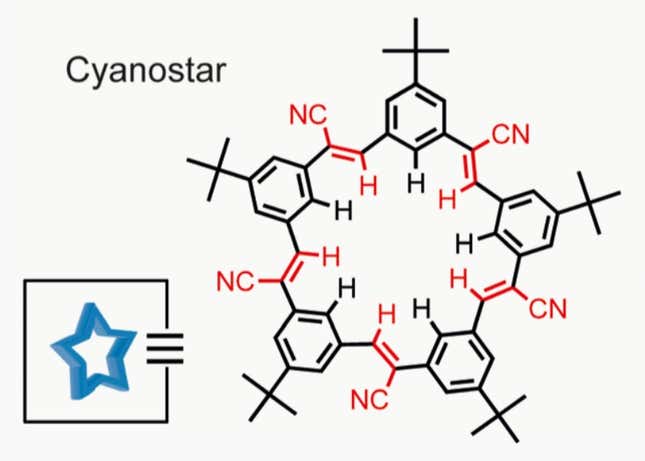
These structures contain an inner ring of hydrogen atoms (the red H’s in the diagram) that are slightly positively charged. The result is pocket of positive charge that can attract negative ions, such as the chlorine in salt.
At least, it should. And usually, it does. In Flood’s experiments, two cyanostar molecules could trap a single negative ion between them, like a ball bearing sandwiched between two rings.
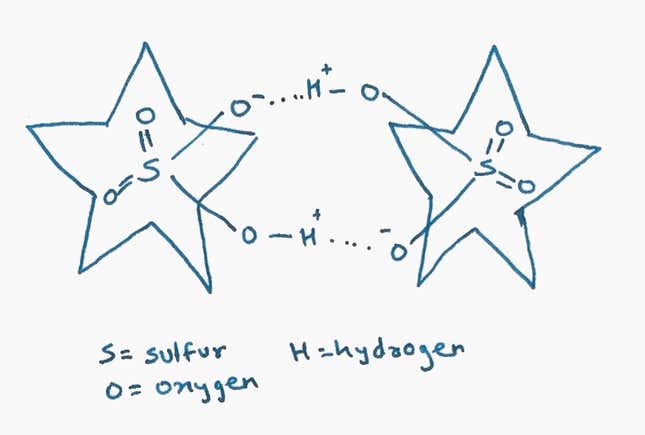
However, with one particular negative ion—bisulfate (HSO4–)—something odd happened. Flood found that the cyanostars had trapped two bisulfate ions between them instead of one. That ought to have violated Coulomb’s law, because it meant two negative ions, which should repel each other, were instead sitting side by side.
“To be honest, I was shocked to find two negatively charged ions sitting so close to each other,” says Flood.
What’s going on? Within the constraints of the molecular cage, Coulomb’s law is in action: the negative charges on the bisulfate ions repel each other. But the positively charged holes in the centers of the cyanostars have the effect of diminishing that repulsion, and that makes it possible for weak chemical bonds (called hydrogen bonds) between the two bisulfates to keep them from flying apart.
This could have industrial applications. In processing of nuclear waste, for instance, radioactive material needs to be treated with caustic soda, and then nitric acid, before it can be vitrified into glass and stored underground. This treatment helps concentrate the radioactive material, reducing the quantity of waste that needs to be stored. The nitric acid contains nitrates, which contain negative ions similar to bisulfate, and these need to be removed from the waste before vitrification can begin. Flood believes that cyanostar-type molecules could help remove them.
Another possible application is in environmental pollution caused by fertilizers. When rain washes these from the ground into streams or lakes, they provided the perfect fodder for toxic algal blooms that can harm other forms of life. With the help of cyanostar-type compounds, Flood believes you could build a detector for negative ions that would warn if the concentration is getting too high. There are other ways to do that, but this discovery could help create a more effective and perhaps cheaper tool.
