Only a handful of countries are backing a life-saving HIV-prevention drug
Medication tends to work best when people can actually take it.
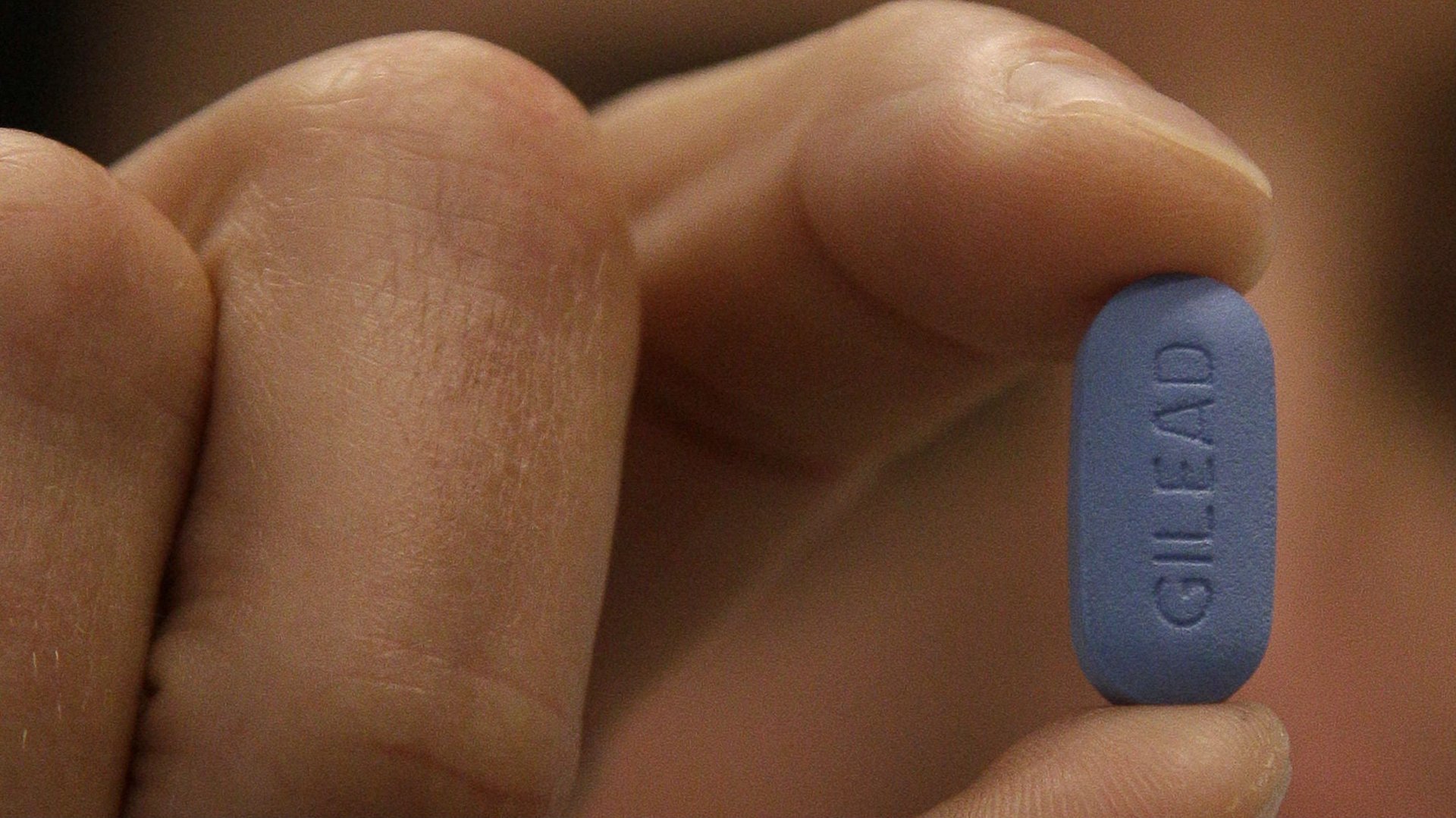

Medication tends to work best when people can actually take it.
Let’s look at Truvada, a pill manufactured by California-based Gilead Sciences that is 90% to 99% effective at preventing HIV infection. Continuous daily use is often referred to as pre-exposure prophylaxis, or PrEP. Truvada—which contains two drugs—stops the virus from infecting someone who encounters it through sex or shared needles. It costs about $1,500 per month, making it too expensive for many who would benefit.
There are a handful of countries that provide subsidies or have other programs in place to make the drug more accessible. Norway, France, Belgium, Kenya, South Africa, and Brazil all have government programs lowering the cost of the drug, and in some cases, making it free. Today (Feb. 7), New Zealand became the latest to join this group; starting next month, a Truvada prescription will be just $50 a month. Australians are hopeful that the government will announce the same kind of support for Truvada this week.
In the US, there’s no federal program to reduce the cost, but New York City, Los Angeles, and San Francisco all provide the medication for free. So do Quebec and Ontario in Canada, and the Canadian federal government has approved PrEP as prophylaxis for HIV in general. Other countries like England are still in the midst of their own clinical trials to ensure the drug is safe and effective.
Although a generic form of Truvada was approved in June 2017, for legal reasons the drug hasn’t been widely used yet. Insurance can cover some of the costs of Truvada, although it varies from provider to provider. These companies are supposed to look at a drug’s efficacy in order to decide to cover it, but even so, not everyone can afford the copay. Gilead offers some resources for people who can’t afford the drug in the US, including up to $3,600 per year in coupons. The Patient Advocacy Foundation, an independent group headquartered in Virginia, covers prescription copay costs on a case-by-case basis. But according to the AP, as of January only about 145,000 people had prescriptions for the drug in the US, when there are over 1 million people in the country who would benefit from it.
US federal guidelines state that PrEP should be given to people who are in a relationship with someone who already has HIV. That said, the US Centers for Disease Control considers those who are in a relationship to be men who have sex with men or use needles to take drugs Some argue that sex workers should be included in that group, too.
“Truvada works,” James Krellenstein, a New York-based AIDS activist, told the AP. “We have to start thinking of it not as a luxury but as an essential public health component of this nation’s response to HIV.”