You could snort your next flu vaccine (again)
In the midst of this awful flu season, it doesn’t feel too early to start prepping for the next one.
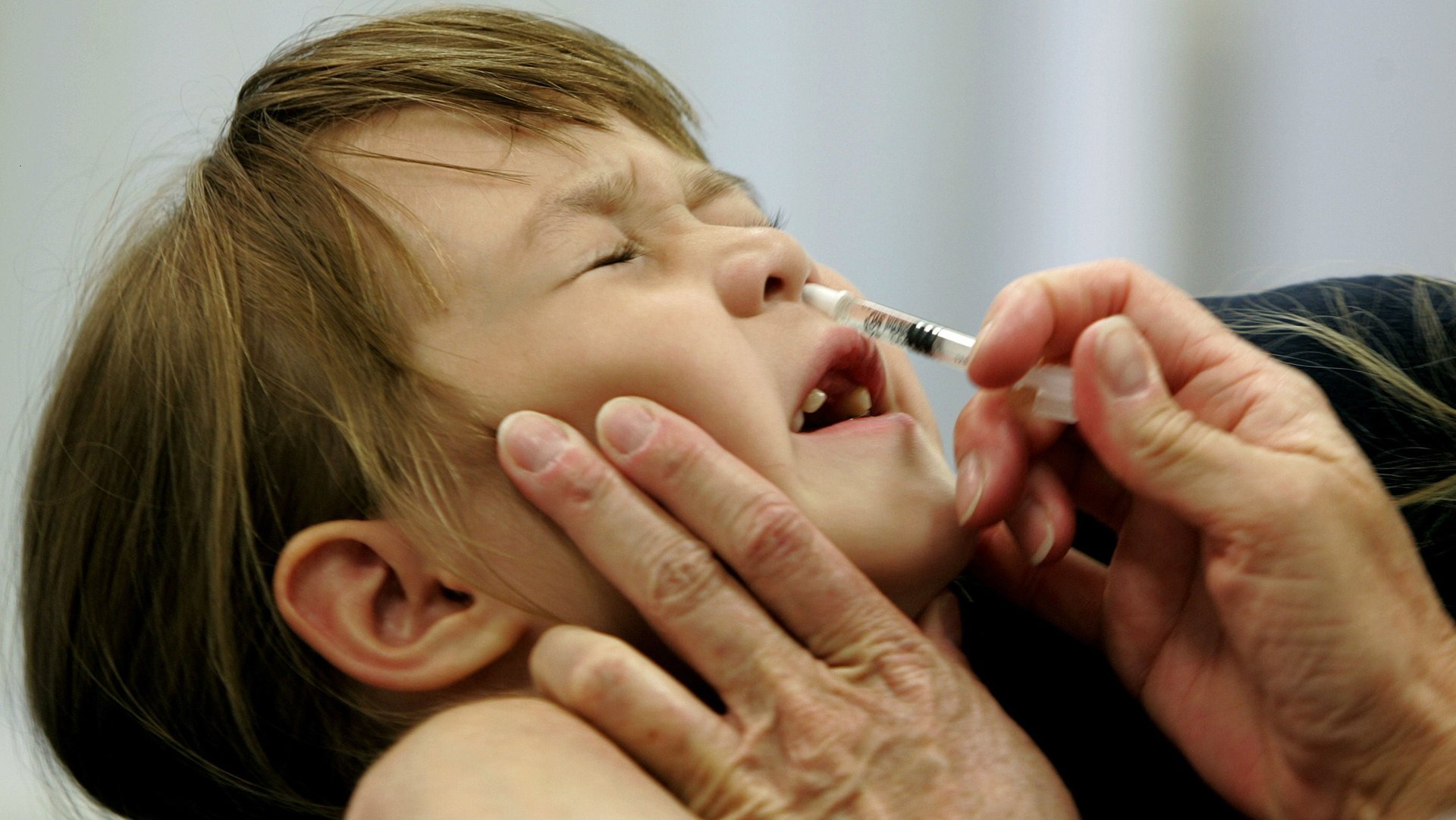

In the midst of this awful flu season, it doesn’t feel too early to start prepping for the next one.
On Wednesday, Feb. 21, the US Centers for Disease Control’s Advisory Committee on Immunization Practices (ACIP) voted 12-2 to once again endorse FluMist, a vaccine for the flu that can be inhaled like an allergy medicine, for the 2018-2019 season. That reverses an ACIP decision to stop endorsing the inhaled version of the vaccine after the 2015-2016 flu season.
Though the ACIP isn’t technically a regulatory body, in practice, it essentially is: Vaccines without the committee’s stamp of approval are hardly sold anywhere. FluMist was made available in June 2003, and for over a decade, it appeared to work reasonably well. As early as 2007, the ACIP had approved its use for children as young as 2 years old. In 2014, the ACIP made a rare “preferred recommendation” that FluMist be used for children aged 2 to 8, because it was easier to distribute in schools than the typical shots. Typically, the committee doesn’t make these sorts of (paywall) recommendations for a specific product.
FluMist hit some road bumps in recent years. During the 2015 season, CDC researchers a found that FluMist was less effective against all flu strains, in large part because it didn’t seem to work at all against the H1N1 strain of the flu virus, also known as swine flu. The ACIP removed its endorsement for FluMist before the onset of the fall of 2016, and distributors stopped buying the vaccine (even though it still had US Food and Drug Administration approval).
MedImmune, the branch of the London-based AstraZeneca that manufactures FluMist, says that since 2015, it has updated the vaccine to improve efficacy against H1N1. Its improvements were demonstrated in company-sponsored testing. But real-world efficacy is a far better measure of how well a vaccine actually works than lab tests, and we haven’t seen another flu season dominated by H1N1 since the 2009-2010 season (though there’s bound to be one in the future).
“The effectiveness of this formulation against [H1N1] is not known, and is likely to remain unknown until the next H1N1 predominant season,” and only if people start using FluMist again now that it has APIC backing, Lisa Grohskopf, a CDC flu specialist, told STAT.
The ACIP’s decision to re-endorse FluMist is less about any improvements in its efficacy, than in the thinking that needle-averse patients like children (and really, does anyone want to get a flu shot?) will be more likely to take an inhaled flu vaccine. This year, 75% of the 84 children who have died from the flu in the US did not receive a vaccine.
As STAT notes, the ACIP endorsement of FluMist doesn’t mean patients will see the sniffable vaccine everywhere next year. Many healthcare providers have already pre-ordered their vaccine supplies for next season, pending the updated formula. But there’s still a chance that next year those wishing to get vaccinated without a needle will have a better luck finding FluMist at other vaccine distribution points, like pharmacies, than they have in the past couple of years.