There are two types of water, and a new study finds they behave differently
It’s not just poets. Chemists also like to wax lyrical about water, because there’s nothing like it. You probably already know some of the cool stuff about water: unlike all other chemicals, water becomes less dense when you cool it from a liquid state to a solid state (that’s why ice floats on water); it’s among the few chemicals not containing carbon that is liquid at room temperature; and, of course, life as we know it wouldn’t exist without water.

It’s not just poets. Chemists also like to wax lyrical about water, because there’s nothing like it. You probably already know some of the cool stuff about water: unlike all other chemicals, water becomes less dense when you cool it from a liquid state to a solid state (that’s why ice floats on water); it’s among the few chemicals not containing carbon that is liquid at room temperature; and, of course, life as we know it wouldn’t exist without water.
But then there’s odd stuff that you likely don’t know about it. For example, this new discovery: not all water molecules behave the same way. To understand why, let’s revisit some of those dreaded high-school chemistry lessons.
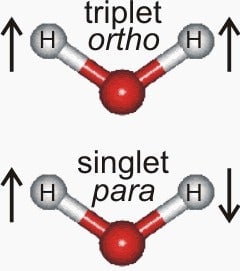
Water is made of two hydrogen atoms and one oxygen atom, linked to form a broad V-shape. Each of those atoms has at its center a rotating nucleus, which can move in one of two direction. When the rotations of the two hydrogen atoms are in the same direction, it’s called ortho water. When the rotations are in opposite directions, it’s called para water.
At room temperature, the two types of water exist in equal amounts. That’s because there’s enough energy in those molecules—room temperature is quite warm, chemically speaking—for each of them to swap back and forth between the different states.
But cool those molecules down (to near absolute zero, −273°C or −459°F), and each molecule stabilizes as either ortho or para, and then you can learn the differences between the two types of water. In a study published in Nature Communications, Ardita Kilaj of the University of Basel and her colleagues have done just that.
Kilaj first used a very sensitive instrument to separate the two types of water molecules at extremely low temperatures. Ortho and para water are are physically almost identical. But because of their different spins, they vary slightly in the electrical fields they generate. The instrument allowed Kilaj to spit out a molecular beam containing both types of water, which, when exposed to an electric field, split in two streams, because of deflections in different directions.
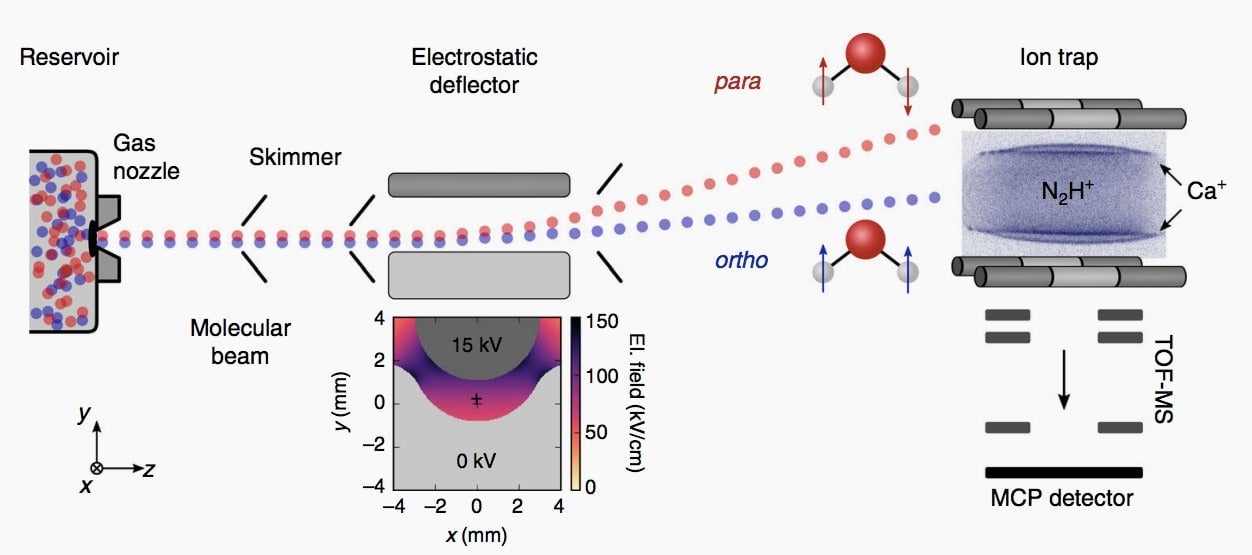
Once separated, each stream of water molecules was pushed into a chamber containing specialized ions called diazenylium, which is a positively charged molecule containing two nitrogen atoms and one hydrogen atom. The ion was chosen because it is among the few types of molecules that can react at all at such a low temperature.
The results showed that para water reacts faster with diazenylium ions than ortho water does, by about 25%. That’s likely because para water’s electrical field is able to attract those ions faster than ortho water’s can. The study is the first known example of how the two types of water can differ in their chemical behavior, according to Kilaj.
What’s the practical use of this knowledge? Not much. “We did the study to understand the basics of chemical reactions,” Kilaj said. “The better one can control the states of the molecules involved in a chemical reaction, the better the underlying mechanisms and dynamics of a reaction can be investigated and understood,” added her PhD supervisor Stefan Willitsch in a press release.
If nothing else, chemists have another oddity for the already long list of odd properties of this life-giving chemical. Not convinced? Try listening to Alok Jha, a science writer who wrote a whole book on water’s oddness: