Biogen’s anticipated Alzheimer’s drug is still months away
Last December, Biogen made an exciting announcement: The company was set to submit an Alzheimer’s drug, aducanumab, to the US Food and Drug Administration early in 2020 and strongly expected approval to be granted. Four months later, there’s been no sign of progress. And in an earning’s call today, Biogen said there would be further delays, with plans to submit the FDA application in the third quarter this year.
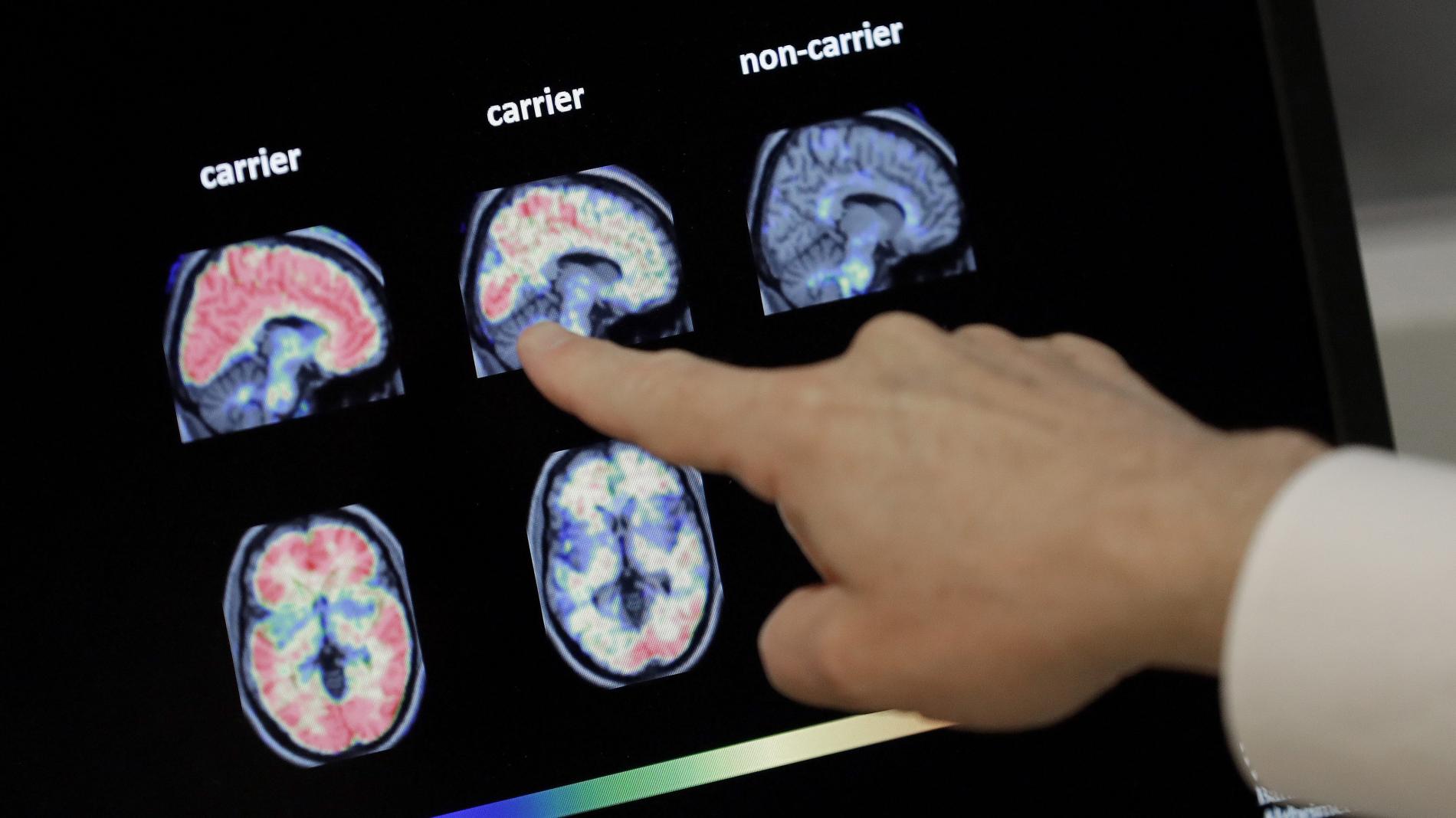

Last December, Biogen made an exciting announcement: The company was set to submit an Alzheimer’s drug, aducanumab, to the US Food and Drug Administration early in 2020 and strongly expected approval to be granted. Four months later, there’s been no sign of progress. And in an earning’s call today, Biogen said there would be further delays, with plans to submit the FDA application in the third quarter this year.
If approved, aducanumab would be the first Alzheimer’s drug that treats the disease directly rather than alleviating symptoms, and would be the first new Alzheimer’s treatment for nearly two decades. But there’s already some skepticism about the drug, which has followed a strange development path: Biogen initially cancelled its aducanumab trials citing insignificant effects, and started them again after it re-analyzed early data.
Despite repeated questioning from analysts, there was little clear explanation for the change in timeline. The company said it would need several more meetings with the FDA this summer before filing for approval. “Timing is not easy to predict,” said Al Sandrock, Biogen’s head of research and development. “Nothing has come up with the data that has changed our interpretation.” Several analysts who previously expected the drug to be approved in 2020 changed their predictions to 2021 or 2022 following the earnings call.
Although Biogen’s timeline is now months longer than the company initially suggested, Sandrock said employees’ personal experience of coronavirus in March contributed to the delay. Biogen’s annual leadership meeting in Boston the first week in March was one of the first “superspreader” events in the US, with employees unknowingly carrying coronavirus to at least six states and three countries. The Massachusetts Department of Public Health attributed 99 cases of coronavirus in the state to Biogen conference attendees and their contacts, as of March 27.
“Some members of the team did get Covid. I can tell you, it’s hard to work when you get Covid,” said Sandrock. He did not comment on how many employees were affected in the company overall, and how many worked directly on aducanumab. “The fatigue, mental and physical fatigue, was such that there were some people affected by this disease.” All employees had recovered or were recovering, said chief executive Michel Vounatsos.
The company acknowledged that coronavirus will continue to cause challenges, including interrupting clinical trials. Biogen said it was planning to continue the “vast majority” of the clinical trials highlighted last year, and would have to turn to remote monitoring, telemedicine, and IV home infusions to continue safely.
One silver lining has emerged from Biogen’s personal experiences with coronavirus: The company is working with the Broad Institute of MIT and Harvard and Partners Healthcare to create a Covid-19 biobank. On the earnings call, Vounatsos said Biogen employees and their friends, many of whom were infected, would be able to donate biological samples and medical data. For once, Biogen employees will be not just researchers, but participants in a clinical study.