Harvard scientists have turned fat cells into lasers
In an achievement that’s as freaky as it is functional, scientists have managed to turn cells into miniature lasers.

In an achievement that’s as freaky as it is functional, scientists have managed to turn cells into miniature lasers.
One way to create a laser is to put dye into an enclosed, reflective space and then bounce light off it. The light that comes out is a concentrated, monochromatic beam—laser light. A team at Harvard Medical School, led by physicists Seok Hyun Yun and Matjaž Humar, has used that same process to build tiny lasers inside living cells by filling them with dye.
Their method could one day be used to tag individual cells, such as cancer cells, and track their movements through the human body. The team detailed the process in a paper published in Nature Photonics.

Yun and Humar used three different methods to create their intracellular “micro-lasers.” In one example, they injected oil into human cells—”almost any cell” will work, Humar tells Quartz—and filled the oil droplets with fluorescent dye. When they shined a light on the cells, the dye atoms within produced a focused beam of light.
In another method, the researchers made cells called macrophages, a type of white blood cell that ingests foreign matter, eat polystyrene beads filled with dye. These too emitted lasers when they were hit with non-laser light.
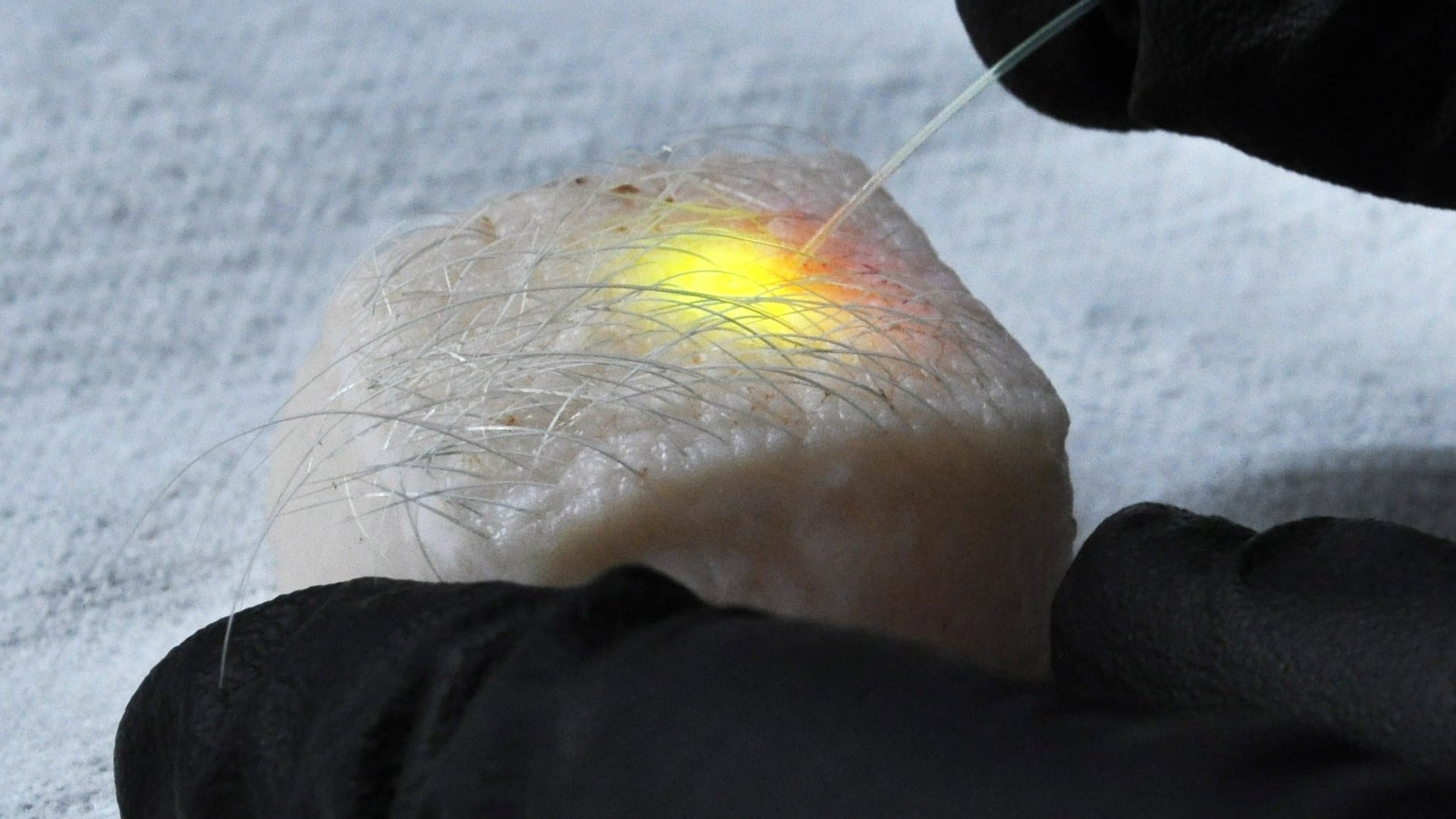
Lastly, the Harvard team used the fat cells of pig skin. As in the previous examples, dye injected into the cells of fat just under the skin’s surface emitted lasers when the team exposed it to light through a subcutaneous optical fiber. (Just shining a light on the skin’s surface won’t do it, because the fat cells are underneath.)
The finding could make for significant improvements in cell imaging, used frequently in research on cancer and infectious diseases.
It’s already common for researchers to tag cells with dye. In current imaging techniques, dyed cells emit a broad range of wavelengths, making it difficult to distinguish one cell from another. Lasers work in a much narrower range, making it theoretically possible for researchers to give each cell an individual laser light signature.
“Maybe the most interesting application is cell tagging, so you can see how the cells move,” Humar told Quartz.
So far they’ve only tagged cells in Petri dishes, but Humar says there’s no reason it wouldn’t work in a living body. “In principle, it could be possible to individually tag and track every single cell in the human body,” he and Yun wrote in a blog post for Discover.
One day, they say, intracellular lasers could even help determine what a cancer cell is made of, by allowing doctors to analyze the interior with the laser light instead of a biopsy.
The Harvard team isn’t alone in its research on turning cells into lasers. As New Scientist reported, a group at the University of St. Andrews in the UK has also worked with using macrophages to create intracellular lasers.