The GSK way: make hard-to-mimic drugs and don’t worry about patents
GlaxoSmithKline (GSK), the British pharmaceutical company, reported lackluster fourth quarter earnings for 2012 this morning, with a 3.5% drop in revenue. But the company’s performance would have been much worse if it hadn’t successfully avoided a looming threat that every brand-name pharmaceutical maker faces from time to time: the end of a patent on a blockbuster drug.


GlaxoSmithKline (GSK), the British pharmaceutical company, reported lackluster fourth quarter earnings for 2012 this morning, with a 3.5% drop in revenue. But the company’s performance would have been much worse if it hadn’t successfully avoided a looming threat that every brand-name pharmaceutical maker faces from time to time: the end of a patent on a blockbuster drug.
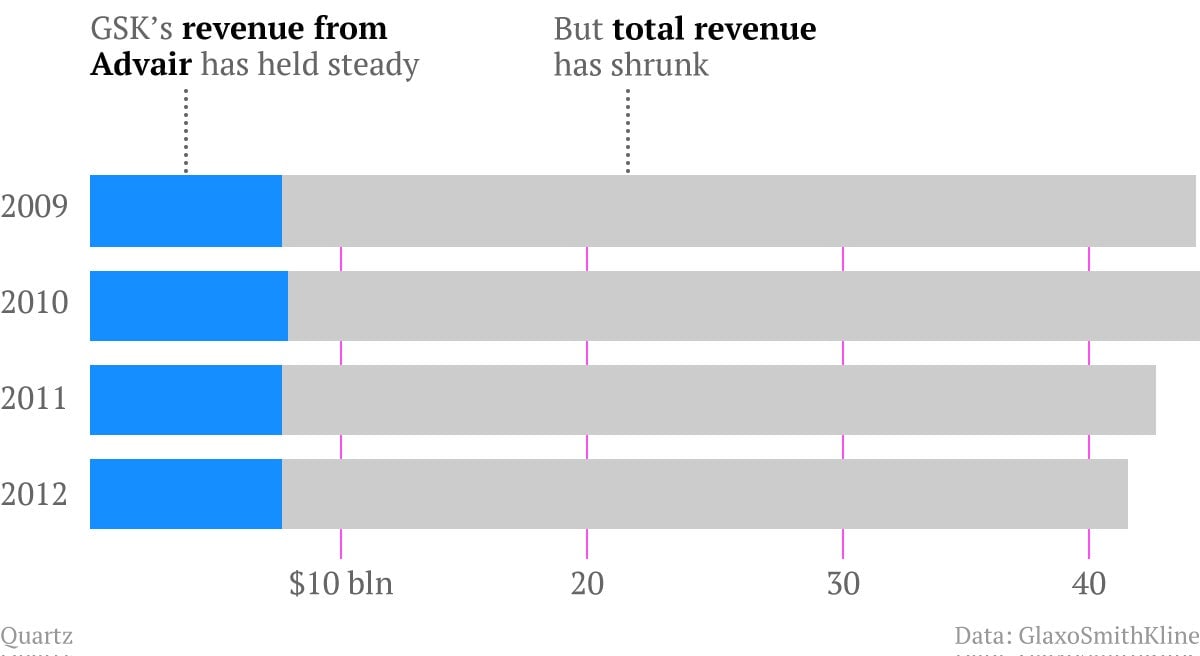
GSK’s Advair inhaler (called Seretide in most of Europe and India)—used to treat asthma and chronic obstructive pulmonary disease—lost its patent at the end of 2010. Ordinarily, a cheaper, generic version of a patented drug comes out shortly after the patent expires, and the generic quickly eats away at the marketshare and revenue of its branded progenitor. But Advair still brings $8 bln in sales to GSK, making it the third highest grossing drug worldwide. The only other off-patent pharmaceutical in the top ten is Lipitor, used for treating high cholesterol, which earned its maker, Pfizer, less than half as much in 2012 as it did in 2011, the year its patent expired (in spite of Pfizer’s unprecedented campaign to keep Lipitor a top-seller by strategically slashing prices).
The reason Advair has been immune to a takeover of generics is that, unlike a basic chemical pill, it’s extremely difficult to mimic. A typical inhaler for treating respiratory problems consists of a pressurized canister full of liquid medicine; when the canister is compressed, the liquid sprays out as a breathable aerosol in an amount that can easily be pre-determined by the manufacturer. Advair, on the other hand, is not a liquid, but a mixture of powders that patients propel into their respiratory tracts using a specially made inhaler called a Diskus.
Large generic makers have made preliminary attempts to replicate the powders and the Diskus with the help of some former GSK employees, but they doubt they’ll be able to make a close-enough copy to satisfy the stringent “substitution” requirements the US Food and Drug Administration places on generics. Teva, the world’s largest maker of generic drugs, announced at the end of 2010 that it would not attempt to make a generic version of Advair for the US but would instead develop its own branded alternative.
In Europe, however, where the regulation of generics is bit more lax, GSK expects competition from off-brand copies of Advair in the coming years. For this reason, and because sales in the region have been shrinking due to austerity-driven cuts in drug prices, GSK announced this morning it will be scaling down its European operations.