Coronavirus: Vax and figures
Hello Quartz readers,

Hello Quartz readers,
The world has at least two Covid-19 vaccines ready to be distributed before the end of the year. In several months, millions of citizens around the world will have received at least the first dose of a vaccine made by Pfizer, Moderna, or AstraZeneca.
Unlike most drugs, vaccines aren’t big money makers. Before Covid-19, the global market for all vaccines was about $24 billion a year—only a very small fraction of the overall pharmaceutical market, with its estimated annual revenues of more than $1 trillion. Flu vaccines, for example, are a $4 billion market with Sanofi, the largest producer, selling about $1 billion per year.
In the case of a Covid-19 vaccine, there is also significant public pressure to keep the cost as low as possible in order to make vaccines accessible globally. This is why pharmaceutical companies have committed to delivering the first several million doses at reduced prices—from as little as $3 per dose (AstraZeneca) to $25 to $37 per dose (Moderna).
With a larger market than the flu vaccine and a smaller profit margin (at least initially), the annual revenues for the Covid-19 vaccine are projected to be $10 billion a year until the vaccine stops being a public health hazard, which might only be a couple of years. By comparison, pharmaceutical company Merck makes over $7 billion a year with cancer drug Keytruda alone.
While pharmaceutical companies won’t really be able to maximize their profits in the short term, they might be able to pull it off once Covid-19 is still hanging around but no longer a global threat. However long that post-pandemic period lasts, it will paradoxically be easier for pharmaceutical companies to negotiate higher prices for their vaccines when they aren’t as essential for the functioning of the world.
Patent spending
There is another way Pfizer and Moderna are likely to make money from the vaccine: patents. A vaccine can have dozens of patents attached to it—the HPV vaccine has 81—that cover everything from the formula to the method of administration and the manufacturing process.
Patents for vaccines aren’t typically that remunerative, because there’s little that hasn’t been patented already. But Covid-19 vaccine development has pushed forward research on using messenger RNA to trigger an immune response. This innovation is likely to be captured by several patents, and could provide longer-term licensing revenues. “The idea is that the companies don’t care as much for the Covid-19 vaccine as for the trigger of the mechanism that makes it effective,” says Ana Santos Rutschman, a professor at the Center for Health Law Studies of Saint Louis University.
👋👋👋
Speaking of patent-pending innovations, have you considered Quartz membership? It’s the best way to support our journalism, and experience everything Quartz has to offer—like this guide to leading employees through change, or this one about how Covid-19 is altering our eating habits, or this one on how science’s great pandemic pivot made all this vaccine news possible.
Take 20% off your first year with the code QZTWENTY, or test it out with a seven-day free trial.

Predictable
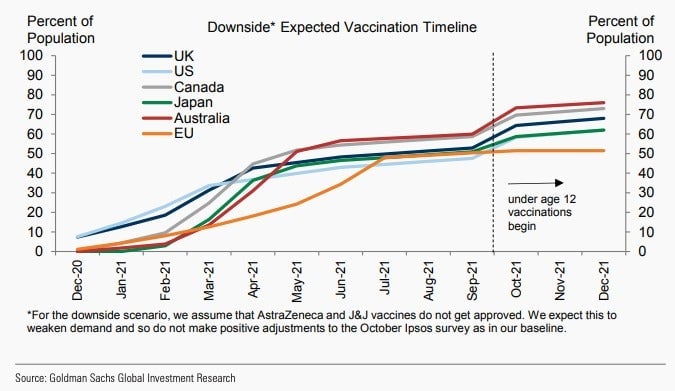
Economists at Goldman Sachs think large swaths of developed countries will be vaccinated by the middle of next year.
In the US, high-risk groups will probably start receiving doses of a vaccine by mid-December, which could provide substantial public health benefits by March, according to a research note by Goldman economists. They expect Britain will have vaccinated about 50% of its population by March, while Canada and the US will have done so in April. The EU, Australia, and Japan will likely reach that point by May, and vaccinations may exceed 70% in all developed economies by the fall, the note says.
Pre-order of magnitude
According to a tally from the Duke Global Health Innovation Center, individual countries and the European Union have already ordered 2.8 billion doses of potential vaccines from Pfizer, Moderna, and AstraZeneca. (Many countries have also pre-ordered vaccines from other companies, but these aren’t yet in late-stage clinical trials.) Should all three of these candidates gain regulatory clearance, six countries—Canada, Japan, the US, Australia, and Chile—plus the European Union could vaccinate more than 100% of their entire populations based on the number of these three vaccines they’ve pre-ordered.
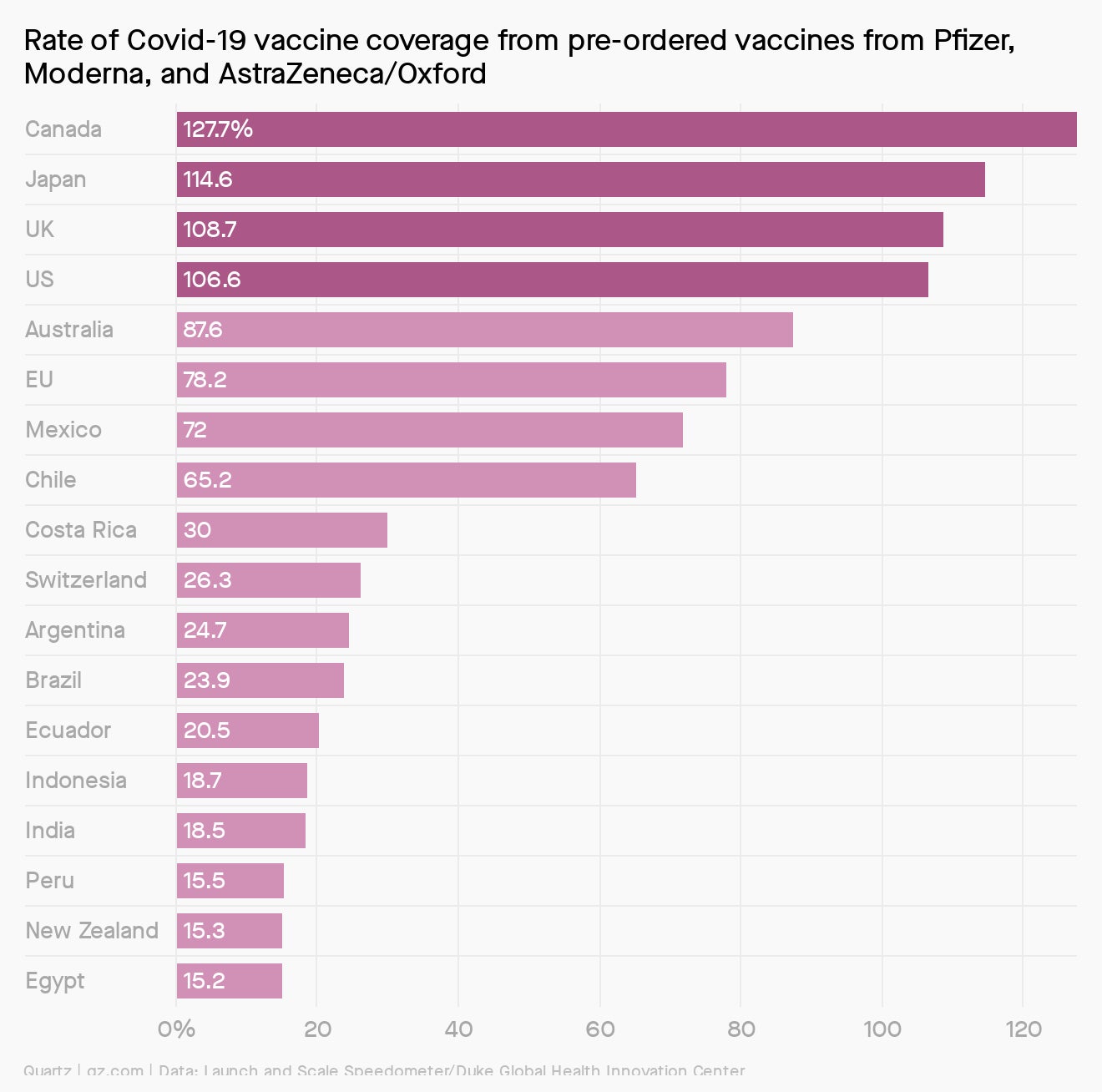
The scramble to pre-order vaccines helped spur their rapid development—essentially, these orders allowed vaccine developers to take on the financial risk of clinical trials without knowing whether the candidate will work—but it also creates a very real possibility that the vaccines will be unequally distributed.
Quotable
“[If we weren’t in the middle of a pandemic, I would be] in Cannes, on a yacht, sailing down the coast with my family, watching the Grand Prix, attending the film festival and spending time with friends there…I suppose I’m a little sad we’re not there at the moment. But the excitement and joy that comes with what I’m doing right now more than compensates. This really is a once-in-a lifetime opportunity.” —Adar Poonawalla, CEO of vaccine maker Serum Institute of India
The 39-year-old billionaire pledged his support for the AstraZeneca vaccine back when the trials were in their first phase, saying his company would manufacture 1 billion doses when the vaccine was ready for market. Poonawalla’s investment toward this goal—$250 million (Rs1,845 crore)—made him India’s most prominent face in the race to procure enough affordable shots. Quartz India dives into the Poonawalla family’s big, brazen bets on the future of vaccines, and the brewing legal battle around its most recent clinical trials.

Essential reading
- The latest 🌏 figures: 63.5 million confirmed cases; 40.8 million classified as “recovered.
- Now in recession: India is faring worse than most major economies.
- Perché: Italy is short on healthcare workers but won’t hire foreigners.
- Hey big spender: Don’t expect Janet Yellen to curb US government stimulus.
- Use your words: The language that actually persuades people on the pandemic.
- Quick Q: Who will get the coronavirus vaccine first?
Our best wishes for a healthy day. Get in touch with us at [email protected], and live your best Quartz life by downloading our iOS app and becoming a member. Today’s newsletter was brought to you by Annalisa Merelli, John Detrixhe, Katherine Ellen Foley, Manavi Kapur, and Kira Bindrim.