Low-background metal: Pure, unadulterated treasure
The search for a clean slate

The search for a clean slate
On July 16, 1945, the world fundamentally, molecularly changed. After the Trinity test, the first successful atomic bomb detonation, governments carried out hundreds of above-ground nuclear tests, filling the atmosphere with radioactive particles. The air we breathe has been a bit more irradiated than normal ever since.
Suggested Reading
One odd consequence of humanity’s 77-year flirtation with atomic weapons is that the steel we produce is now ever-so-slightly radioactive. The radiation isn’t strong enough to pose a health risk, but it does interfere with some sensitive scientific and medical equipment. It’s hard for a geiger counter to accurately measure radiation if the metal it’s made from is, itself, radioactive.
Related Content
This quirk of the nuclear age has created a lucrative but sometimes controversial industry for steel produced before 1945 and centuries-old lead—often sourced from shipwrecks. These “low-background” metals (referring to their low levels of background radiation) have played a key role in treating patients at hospitals, advancing the field of physics, and exploring the cosmos.
Join us on a journey from the bottom of the ocean to the depths of space.
By the digits
528: Above-ground nuclear tests since 1945
2.4 mSv per year: Worldwide average per-capita dose of natural radiation from sunlight, ground deposits of uranium, potassium, and thoron, and so on
0.11 mSv per year: Peak per-capita dose of radiation due to global fallout from atmospheric nuclear weapons testing in 1963, the year the Partial Nuclear Test Ban Treaty (pdf) was enacted
0.005 mSv per year: Per-capita radiation from nuclear weapons testing fallout in 2008
$33 per kg: Price of low-background lead ingots salvaged from the 300-year-old wreck of the Spanish galleon San Ignacio
$1.87 per kg: Today’s spot price for regular lead
Explain it like I’m 5!

Newly made steel and newly mined lead are both radioactive, but for two very different reasons.
Most steel is made by blowing large quantities of air or pure oxygen into molten pig iron (contains no pigs) to remove impurities from the metal and make it stronger. But since the start of atmospheric nuclear tests, all air—and even purified oxygen—contains elevated levels of long-lasting radioactive isotopes like cobalt-60 (pdf). As a result, most new steel becomes infused with radioactive particles from the air.
Lead naturally always contains some amount of the radioactive isotope uranium-235, which decays into a radioactive version of lead called lead-210. When lead ore gets mined, refiners strip away all other elements, including uranium. But the lead they’re left with always contains some amount of lead-210 isotopes, which take decades or centuries to decay into regular, non-radioactive lead. Very old lead, like the metal mined by ancient Romans, has had enough time for its radioactivity to wear off. But newly mined lead will always be radioactive.
Brief history
50-80 BCE: A Roman ship carrying several tons of lead ingots sinks off the coast of Sardinia. Four metric tons of lead from the wreck will one day be used by Italian physicists to build a particle detector.
1733: A Spanish treasure fleet sinks in a hurricane off the coast of Florida. Among its most valuable booty: its lead ballast, which will be recovered and used as shielding for a physics experiment at the University of Chicago.
1919: Rather than turn its ships over to the British, the defeated German Navy scuttles its High Seas Fleet in Scapa Flow, Scotland. Steel from their hulls is used to build radiation detecting equipment at a Scottish hospital.
1945: The US conducts the first nuclear test at the Trinity site in the New Mexican desert.
1963: The US, UK, and Soviet Union sign the Partial Test Ban Treaty banning above-ground nuclear weapons tests. Atmospheric radiation peaks.
1966: Steel cut from the decommissioned ship USS Indiana is used to build radiation detecting equipment at an Illinois hospital.
1980: China—which, along with France, refused to sign the Partial Test Ban Treaty—conducts the world’s last above-ground nuclear weapons test.
Million-dollar question
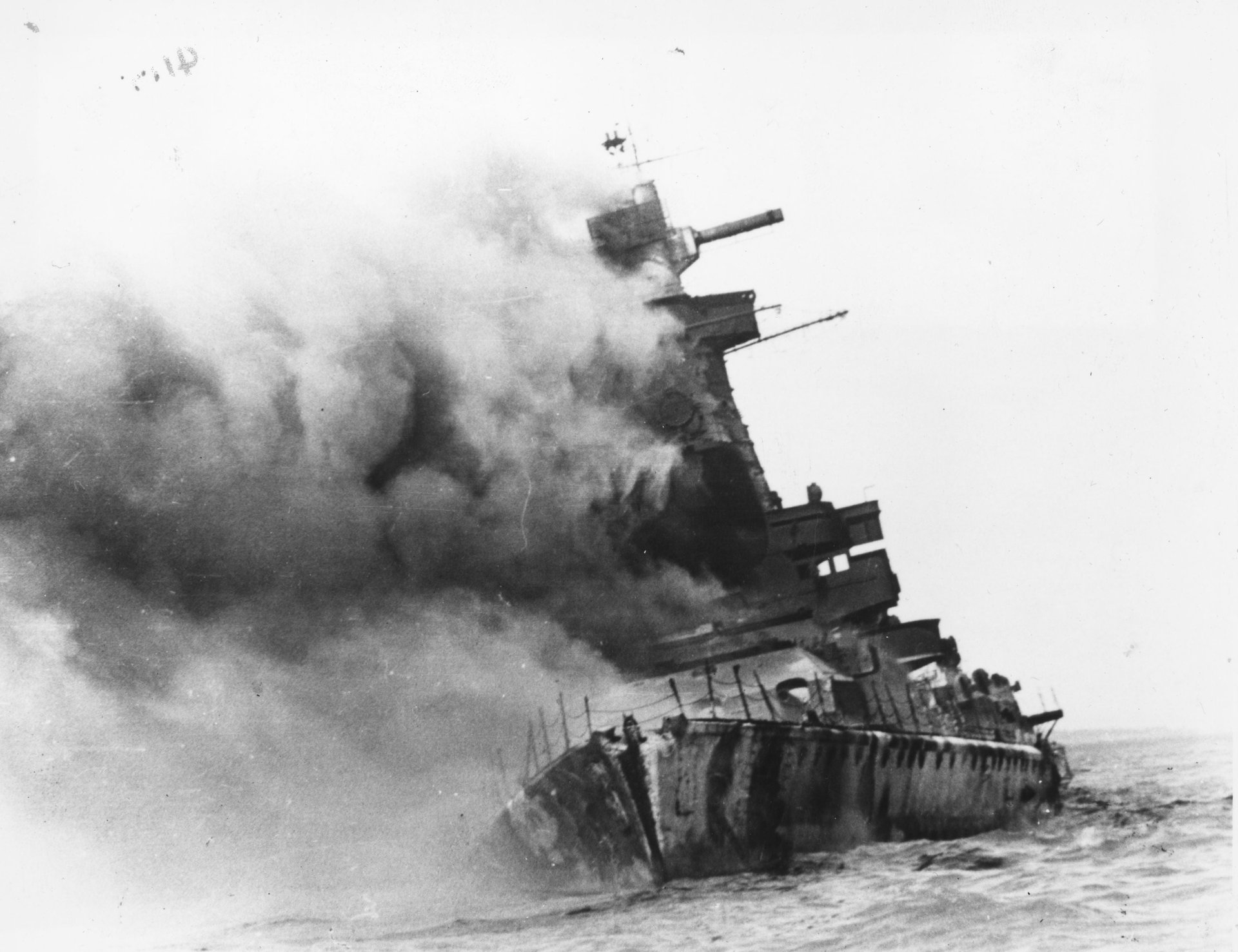
Most low-background steel comes from the hulls of battleships sunk before 1945. The most famous source is the German World War I naval fleet sunk off the coast of Scotland along a flat, shallow stretch of seabed known as Scapa Flow. In 1919, the victorious Allies ordered the defeated Germans to surrender their naval fleet to British authorities at Scapa Flow. But the Germans sank their own ships to keep them out of enemy hands. Salvagers have since harvested low-background steel from the hulls of several of the scuttled ships.
Low-background lead, however, tends to come from the cargo holds of even older ships. The Roman empire used lead extensively to line pipes, baths, and cooking vessels. In 2010, the Italian National Archaeological Museum gave 120 lead ingots recovered from a Roman shipwreck (ca. 50-80 BCE) to the country’s National Institute of Nuclear Physics to use as radiation shielding for a cutting-edge particle detector. The transfer created a minor dust-up between archaeologists and physicists over the prudence of melting down ancient artifacts—still bearing inscriptions from the Roman forges that molded them—to build high-tech physics experiments.
In addition to shielding particle physics experiments, low-background steel and lead are also crucial shielding materials for “whole body counters”—hospital equipment used to measure the level of radiation in the human body if a person has been exposed to radioactivity. These sensors are usually housed in small rooms surrounded by about 20 cm of low-background steel and another centimeter of low-background lead to block outside radiation from interfering with the results.
Quotable
“Are these experiments important enough to destroy parts of our past, to discover something about our future?”
—Elena Perez-Alvaro, postdoctoral fellow at Nelson Mandela University, who wrote a paper (pdf) examining the benefits and drawbacks of using Roman lead for physics experiments
“These experiments can reveal some of the most fundamental properties of the universe, and answer questions such as what are we and where we come from. I think it’s worth it.”
—M. Fernando Gonzalez-Zalba, a University of Cambridge physicist who collaborated on Perez-Alvaro’s paper
The way we ⛏️ now

The level of atmospheric radiation from nuclear testing has fallen roughly 95% from its peak in 1963, as above-ground nuclear tests slowed to a halt and radioactive particles in the air have gradually decayed out of existence. The steel we produce today emits much less radiation than the steel we produced a few decades ago (although it’s still a bit more radioactive than pre-1945 steel). Meanwhile, scientists have found ways for some radiation-sensing equipment to work despite the small amount of interference it might receive from radioactive steel parts.
As a result, low-background steel sourced from pre-1945 shipwrecks has become less valuable. But low-background lead from ancient sources remains important, because the lead we mine today is still radioactive. All freshly mined lead will always be radioactive, because when it’s in the ground, it will always be mixed up with uranium-235 that is constantly decaying into radioactive lead-210. So the market for ancient low-background metal will likely never go away.
Fun fact!
Steel from Scapa Flow is rumored to have been used in making the Voyager satellite that is now traveling to the edge of the solar system, although NASA has never confirmed this claim.
Watch this!

Up to 40 World War II shipwrecks have disappeared in recent years, as salvagers ransack them for low-background steel and other valuable parts, like copper wiring and bronze propellers. Unlike the Scapa Flow ships, however, many of these are protected war graves that still contain the bodies of drowned sailors, leading to outcry over the illegal salvaging operations that have caused the wrecks to disappear. This video will take you there.
1,000 words

Randall Munroe, the NASA-contractor-turned-cartoonist who writes the long-running xkcd webcomic, devoted his 2,321st cartoon to low-background metal. (If you don’t get the joke, you can always consult the online encyclopedia dedicated to explaining Munroe’s sometimes inscrutable comics.)
Poll

Would you melt down ancient lead to build cutting-edge physics experiments?
We must know—our entire future could be at stake! Or our past! Depending on how you want to look at it!
💬 Let’s talk!
Today’s email is the last in a long line of Obsession collaborations written by Nicolás Rivero (moderately radioactive) and barely edited at all by Susan Howson (shedding slightly radioactive tears).