India has approved the world’s first DNA vaccine for Covid-19 without any trial data
India has added another homegrown Covid-19 vaccine to its basket—and once again without official trial data.
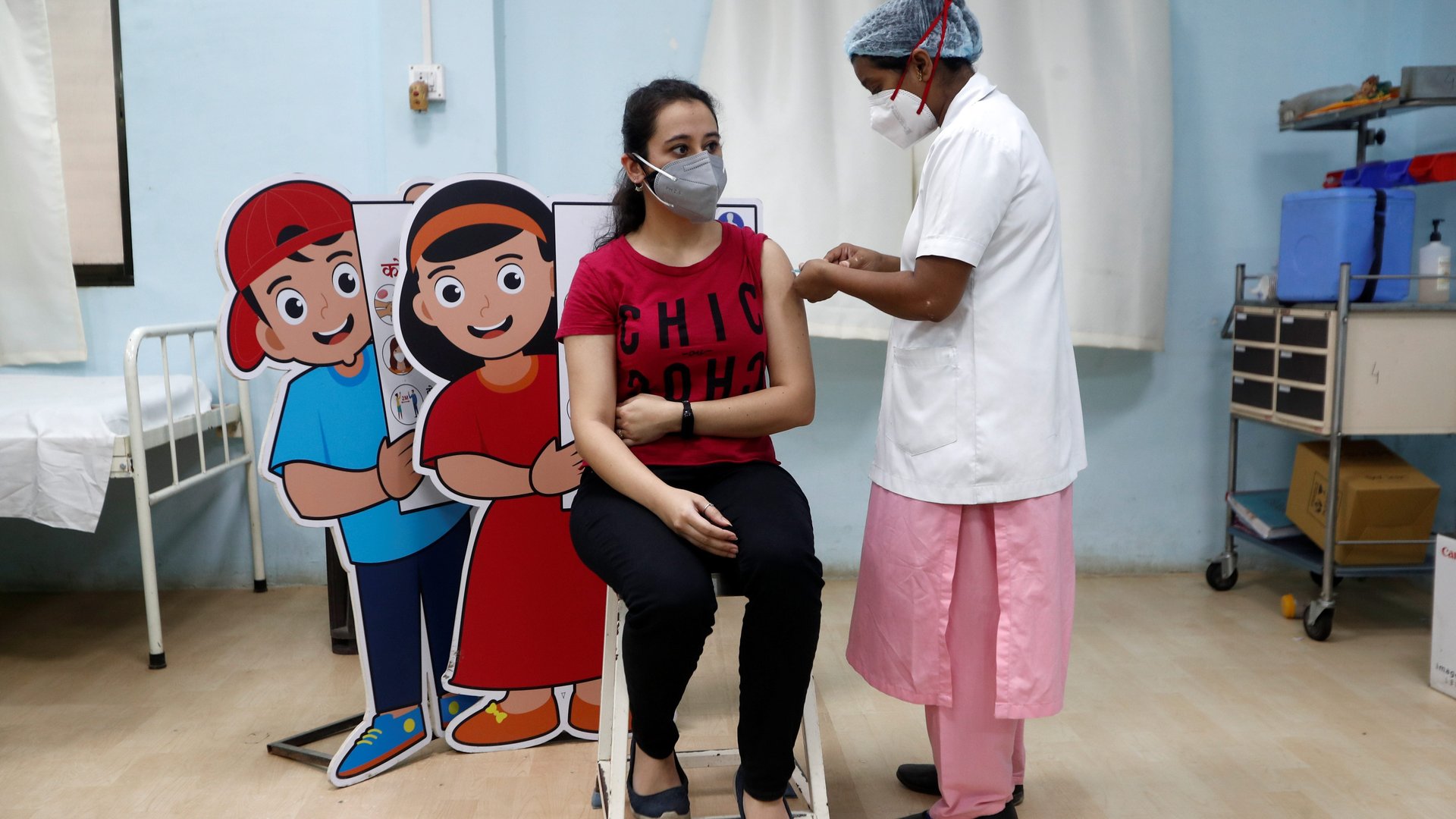
India has added another homegrown Covid-19 vaccine to its basket—and once again without official trial data.
On Aug. 20, India’s central drugs regulatory authority approved the ZyCoV-D vaccine, developed and manufactured by Zydus Cadila. The company has claimed that the three-dose vaccine, built on the plasmid DNA platform, has 66.6% efficacy against symptomatic Covid-19. It also claims that this vaccine is safe for children over the ages of 12, making it the paediatric option in India.
The vaccine is an innovative leap for India, which has been the first to approve a DNA platform shot for Covid-19 in the world.
However, no such data are publicly available yet, either in a published study or a preprint interim analysis. This is the second time the Indian government has given the nod to a vaccine without phase 3 trial data after approving Bharat Biotech’s Covaxin in January.
Zydus Cadila is an Ahmedabad-based company, and its stock rose nearly 7% on the BSE when the markets opened today (Aug. 23).
What is a plasmid DNA vaccine?
The DNA platform is not new per se and research has been ongoing for several years to develop vaccines using this technology for infectious diseases like HIV or some cancers. At the moment, 11 of the 112 Covid-19 vaccines being researched in the world are testing the DNA platform.
A DNA vaccine essentially uses the plasmid—the small ring-like part of the DNA molecule—to deliver key information to the immune system. This information is supposed to train the body to fight an infection.
In the case of Covid-19, spike proteins are this key information that the plasmid vaccine wants to deliver to the nucleus of a cell. The plasmid, when successful, should be able to trick the body into creating parts of the spike protein found in the novel coronavirus, and then trigger the body’s immune system into generating antibodies, like a well-executed fire drill.
In that it uses the body’s genetic code to deliver this signal to the cells, the DNA vaccine is similar to the Pfizer and Moderna mRNA vaccines. The difference is that the mRNA vaccines don’t need the immune signal to reach right inside the nucleus of the cell, which makes them highly effective.
In principle, both DNA and mRNA vaccines can be tweaked to deal with more dangerous virus mutations like the delta variant.
The most obvious advantage that ZyCoV-D has is that it can be stored between 2º and 8º Celsius. Covid-19 vaccines like Pfizer’s and Moderna’s need ultra-cold refrigerators for storage and transportation.
It also uses a novel vaccine delivery platform called Tropis, which is PharmaJet’s needle-free injector. This involves an applicator that delivers the liquid through the skin to the proper layer of the tissue.
But DNA vaccines have been successful in animal studies in the past, but have often failed to produce desirable and long-lasting immunity in humans, Indian vaccinologist Gagandeep Kang told the BBC.
This is also why the safety, immunogenicity, and efficacy data are imperative to truly evaluate if the vaccine works against Covid-19.
Zydus Cadila’s missing data
The only information available about ZyCoV-D is through its entry in the clinical trial registry, which is not sufficient at the moment. The phase 3 human trials had 28,000 participants, of which 1,000 were children between the ages of 12 and 18, according to the company’s July 1 press release (pdf).
A May 2021 study (pdf) by Transparency International Global Health, a UK-based non-profit that works against corruption in the pharmaceutical sector, found that Zydus Cadila had announced no trial results. “We found 86 clinical trials are registered across the 20 COVID-19 vaccines. At least some clinical trial results had been announced for 18 vaccines, meaning that two vaccines developed by AnGes and Zydus Cadila had announced no trial results,” it noted.
Such lack of transparency has persisted despite the public outcry against similar concerns for Covaxin, which was given emergency use approvals with no official trial data. ZyCoV-D appears to have even fewer details out in the public domain, especially for a vaccine developed on a new platform.
“In sum, ZyCoV-D may well be a remarkable innovation story for Indian science—but remarkability does not mean it can remain immune to demands of transparency and approval after due process, with publicly available data,” Dr Jammi Nagaraj Rao, a public health physician, independent researcher, and epidemiologist in the UK, wrote in The Wire. “In fact, once again, we are on the cusp of a success story being turned into a blemish on the face of Indian science.”