The teenager inventor who could change the way the world fights climate change
Greenwich, Connecticut


Greenwich, Connecticut
Ethan Novek speaks fast and insists on giving you every detail, even in response to simple questions. It can be overwhelming. But it’s worth sticking with him. Novek started winning science fairs in middle school and was awarded his first patent at 16. Now, at 18, he has his own company, Innovator Energy, and is working on a technology he believes could help dial down global warming.
Despite the growing use of renewable energy, more than 80% of the world’s energy still comes from burning fossil fuels and will continue to do so for decades to come; we simply won’t be able to replace it with renewable energy fast enough. At the same time, under the Paris climate agreement, the world needs to cut emissions fast, in order to reach net-zero emissions by about 2060. If it works, Novek’s technology would allow us to keep burning fossil fuels, without the climate-changing emissions, until we’ve found more sustainable options. The process would still produce carbon dioxide, but the greenhouse gas would be either buried deep in the ground or converted into a useful product.
It’s the sort of world-changing technology that you might expect to come from a well-funded government lab, a startup with deep-pocketed investors, or one of the major players in the billion-dollar energy sector. There’s obviously no guarantee some kid from Connecticut will save the world. But there’s a good reason to pay attention to this particular teen.
Novek approaches all problems starting from first principles. It’s the way we’re all taught to solve problems in school using lessons from basic science, which most adults forget as they get older. But those that do remember can use the method to great success; it’s more or less how Elon Musk approaches problems.
It’s helped Novek identify—correctly—the most valuable link in the carbon-capture technology chain. And his technology, validated through work done at Yale University, attacks that link’s weaknesses in ways many well-established players in the field, including the world’s largest oil companies, haven’t done before.
This article is part of The Race to Zero Emissions series investigating carbon-capture technology. You can also read our feature laying out the case for using the technology to fight climate change.
An early start
I met Novek in Greenwich, Connecticut, on a warm day in September. We’d spoken on the phone before, so I was prepared for how much he loves to hold court. The only difference between Novek on the phone and Novek in person is that, in real life, I could see that he almost never stops smiling.
Novek was born to Keith and Bonnie in a town just outside Boston, Massachusetts. Because of his dad’s work as a consultant, the Noveks moved seven times before Ethan turned eight. Finally, they settled in Greenwich, a famously wealthy town in one of the richest counties in the US. It’s full of celebrities and powerful business people, living in large, lavish homes and commuting into New York City when they want.

“Ethan is an ideas guy,” says his dad, a partner at the global consultancy firm EY. “He’s always carrying his ‘inventions book.’” Ethan got his start in sixth grade, winning a local science-convention competition where the restrictions were to build something that cost less than $25 and could fit on a table. His winning device could capture wind energy released by air vents and convert it into electricity. He called it “ventricity” and it got him featured on the convention’s magazine cover.
“I’ve always been fascinated by energy,” says Novek. “There’s so much of it around us, and my early inventions were attempts to find new ways to capture them.” (He calls them inventions, but most only exist as novel ideas.)
Novek’s first patentable idea came to him while goofing off at the beach in 2014. He was digging a hole in the sand, far from where the waves were crashing. Over time, water started seeping out of the sand and filling up the hole. As the tide crept up, the water in the hole rose. What if, he thought, there was a way to capture the natural energy in these rising waters?
Capturing the energy of ocean tides is not a new idea. The world’s first tidal power plant was built in France in 1966. The concept is simple: Build a barrage to dam up ocean water, creating an estuary. Along the barrage, place turbines that turn as water from the sea flows into the estuary at high tide and when it flows back out into the sea at low tide. Tidal power hasn’t caught on in a meaningful way, though, because it’s expensive to maintain underwater equipment and the turbines are dangerous for fish and other ocean life.
On the beach that day, Novek thought he had a solution. If the water levels in the sand pit were rising and falling at the same pace as the waters in the ocean tide, then theoretically, a turbine placed in the pit could capture the energy of the tide with no impact on fish, because none would be able to pass the through the sieve of sand. Novek filed for and was a granted a patent for the idea.
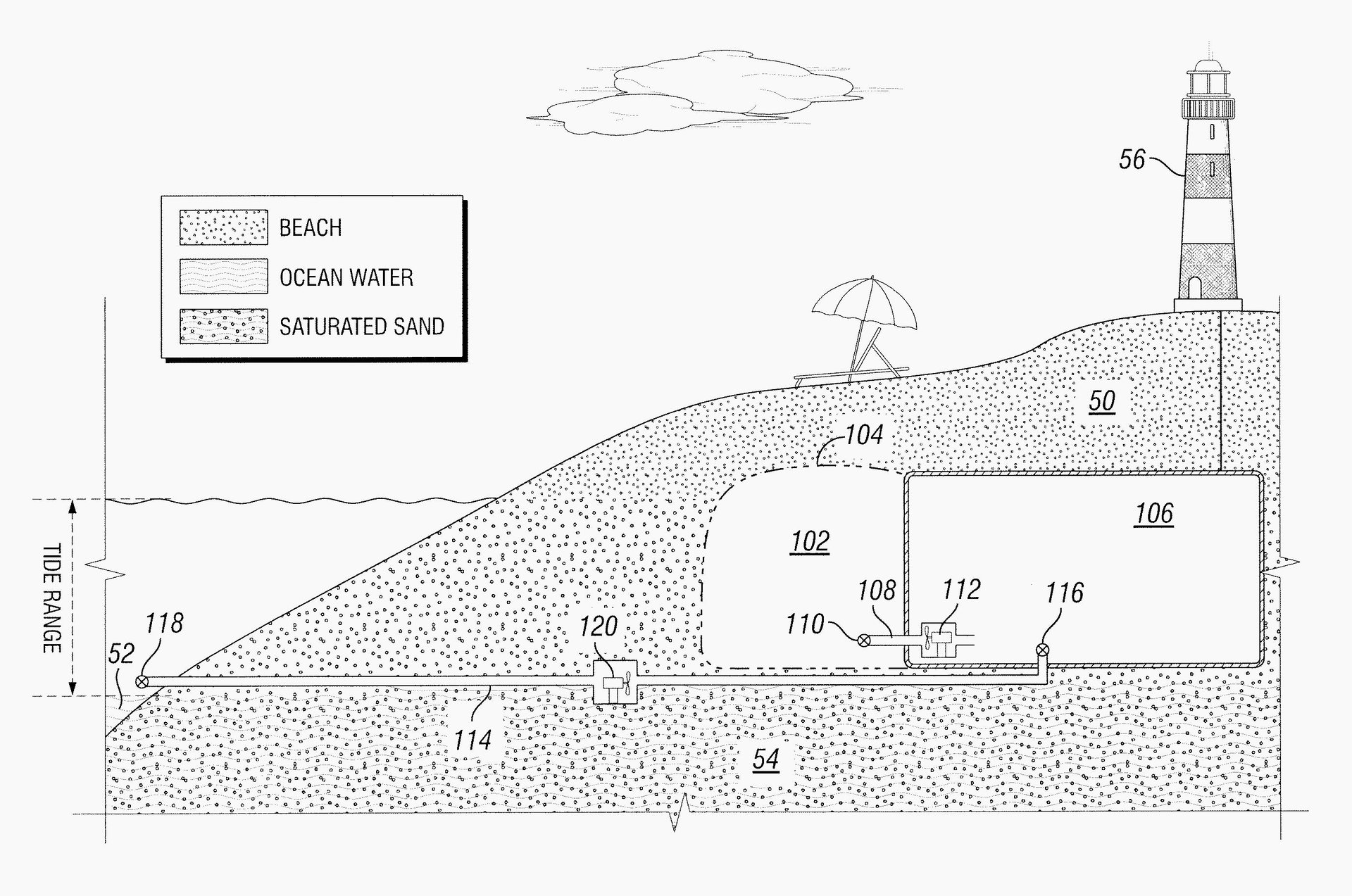
Soon after, however, Novek realized that because tidal power is so low density, he would’ve had to build something quite large to show his idea would work. And after recalculating, he estimated that even with a large system, there were only a few parts of the world where the natural tides rise and fall enough to generate enough energy to justify the expense of construction and operation.
He didn’t despair. “Failure is a good thing,” he says. “It’s the best way to learn.”
Good teachers matter
Standing on the balcony of their large home overlooking a multi-acre garden with a private pond, Bonnie Novek explains how Andrew Bramante’s science-research class at Greenwich High School may have saved the Novek family home.
Before he was accepted into Bramante’s class, Ethan had been doing all sorts of experiments at home. “I had assumed that everything he was doing was ok, but it was good to know he would have a science teacher looking out for him,” says Bonnie. “I was happy that he wasn’t going to blow up our house.”
I visited Bramante’s student research lab at Greenwich High, and if I hadn’t known I was in a high school, I would’ve thought it was a chemistry lab in a top university from the 1990s. Though old, the scientific instruments on offer were more sophisticated than I had ever seen in any high school. Before becoming a teacher, Bramante trained as a chemist and worked for a maker of scientific instruments. There, he became familiar with not just how to use different types of machines but also how the electronics make them work. He now uses that knowledge to repair old, discarded scientific instruments donated to him by pharmaceutical companies and university labs.
The students in Bramante’s class have access to the lab. Getting in requires thinking outside the box. “To get in my class, you have to come up with your own research idea,” says Bramante. “If you give the kid the idea, they kinda sputter out. So it has to be from within.” (His work is set to be featured in a book called “The Class” by former CBS producer Heather Won Tesoriero.)
Once you’re in, you can nerd out as much as you want. “Mr. B gave me a lot of autonomy,” Novek says. “He was there opening the lab for me in the evenings, on weekends, and even during vacations.” Novek was working in Bramante’s lab when he made an accidental scientific discovery that would change his life, and could change ours.
Chasing carbon
When fossil fuels are burned, the exhaust contains a mixture of gases: nitrogen and oxygen, both benign, and carbon dioxide, a dangerous greenhouse gas. To stop that CO2 from entering the atmosphere, conventional carbon-capture technology separates out the exhaust gasses through a process called reversible absorption.
It involves using a substance—usually an amine, an expensive derivative of ammonia—that selectively reacts with only CO2, and other non-greenhouse gases escape. The substance is then moved to a chamber where it is heated, which breaks the bond with CO2 and releases a pure stream of the greenhouse gas that can then be converted into a useful product or buried underground. The absorbing chemical is then put through a new cycle to capture more CO2 and on goes the loop.
This sort of carbon-capture technology has been in commercial use since the 1970s, but it hasn’t been adopted at the scale we need to mitigate climate change, because of four reasons: First, the amines typically used to selectively separate carbon dioxide are expensive. Second, a lot of heat is needed to break the bond between the amines and carbon dioxide, adding additional energy costs. Third, the apparatus in which these reactions are carried out have to be built to high, costly specifications. Finally, environmental groups loathe it because it extends the use of fossil fuels. That’s made for poor public relations. Politicians find it hard to convince people that carbon capture is worth it; even though the technology cuts emissions and can help hit climate goals, at the end there’s no shiny solar panel or wind turbine to show for it.
In his high-school lab, Novek made a discovery that could solve (or at least mitigate) many of these problems. At the time, he was working on something to submit to the prestigious International Science and Engineering Fair (ISEF). He thought he had a killer idea: a new way to cheaply produce urea, one of the world’s most important nitrogen-based fertilizers.
The chemistry to make urea is relatively simple: mix ammonia (NH3) with carbon dioxide (CO2) to get urea (NH2CONH2) and water (H2O). But the chemical reaction only happens under high pressure and at high temperatures, which means it requires a lot of energy. Globally, the production of nitrogen-based fertilizers accounts for slightly more than 1% of all the world’s energy. Any dent to that could have a large impact on the industry’s carbon footprint.

Novek had recently learned a new concept called “salting out” in an advanced chemistry class. He believed he had an idea for a new way to deploy the concept, that would lower the cost of urea production.
In order to study a chemical, you usually need to separate it from the mixtures in which it’s found. Say you’re trying to extract a naturally occurring compound for use in a perfume or cosmetic. Maybe it’s found in the bark of a rare tree. What you’d probably do is put that bark in a solvent, often something as simple as water. After a while, the water-soluble compounds in the bark will seep into the water. But then you’d need to separate those compounds from the water. Distillation is one of the most common methods used to separate compounds; it involves heating the mixture and, because each component of the mixture has a different boiling point, each can be selectively removed from the mixture as temperatures rise.
But in some cases, the compounds are too delicate. In the perfume and cosmetics industries, distillation isn’t always preferred, because the heat causes many of the valuable, sensitive compounds they need to degrade. “Salting out” is an alternative that uses less energy. The charged particles in salt usually like water more than they like whatever water-soluble compounds are in the mixture. As salt is added, the particles break the weak chemical bonds between water and those compounds. Slowly, the compounds starts separating from the mixture.
Novek wanted to see what would happen if he mixed ethanol with ammonium bicarbonate, a salt whose components are ammonia and carbon dioxide. He thought maybe it could break ammonia and carbon dioxide apart and then recombine them, hopefully to produce urea. When he started the experiment, nothing happened. So he heated the mixture to agitate the molecules even more. He was surprised to see a gas bubbling. That didn’t make sense: urea is not a gas.
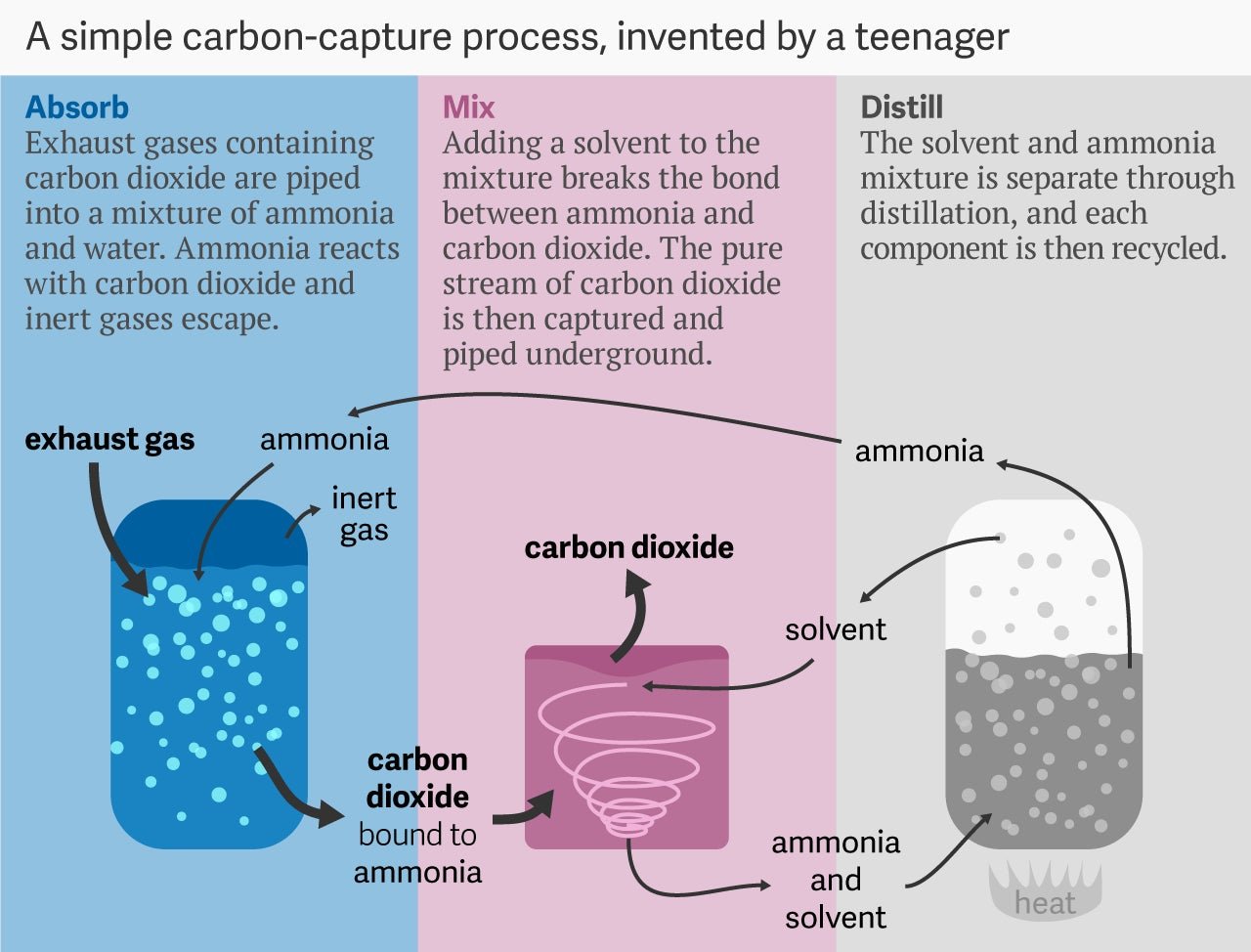
When he tested the gas, Novek realized it was almost entirely CO2. That’s when it struck him: He could use a version of the system to separate out the CO2 that results from burning fossil fuels, and capture it—at a lower cost lower than what the industry can achieve today. The most energy-intensive step in carbon capture is using heat to break the bond between an amine and carbon dioxide. Novek, in his experiment, had just broken the bond between ammonia and carbon dioxide, without very much energy.
Here’s how Novek imagined a future carbon capture system would work: First, exhaust gases containing carbon dioxide are piped into a mixture of ammonia and water. Ammonia reacts with the CO2 to form a salt, and the remaining inert gases (such as oxygen and nitrogen) escape. Second, a solvent is added to the mixture, and breaks down the salt back into ammonia and CO2. The resulting pure stream of carbon dioxide is captured and piped underground. Third, the solvent-and-ammonia mixture is separated through distillation, and each component then recycled through the process.
Early to university
Each year thousands of students around the world win science fairs. Few, if any, take their ideas any farther. Novek was that exception.
As he developed his urea project, Novek kept coming across research papers with the name of a Yale University professor, Menachem Elimelech. Novek emailed him many times seeking advice, requesting access to advanced equipment, and trying to set up an in-person meeting. But he got no response. Finally, after he won a number of prizes at the 2015 ISEF, Novek sent Elimelech a long email with all the new things he had developed in the year he’d spent in Bramante’s lab. This time, Elimelech replied with a one-line response asking Novek to meet in person.
It changed everything. Elimelech liked Novek’s ideas and invited the 16-year-old to join his lab. The Yale professor recruited other researchers to help Novek. The result of their work was a peer-reviewed study published in the journal Environmental Science & Technology Letters in July last year. If everything were to work as planned, Novek’s technology could capture carbon dioxide at $10 or so per metric ton, about 85% less than industry standard.
While experts were reviewing the paper, Novek was busy applying to take part in the Carbon X-Prize, a competition aimed at finding the most effective carbon-capture technology with prizes worth $20 million to be given.
Novek’s application made the cut, one of 22 teams to become an X-Prize semi-finalist. Now, Novek would have to show his technology worked outside of the lab. As part of the next round of the Carbon X-Prize competition, he had 12 months to build a pilot plant that could capture 200 kg of carbon dioxide per day from the exhaust gases of a power plant.
After evaluating quotes from three different places, Novek settled on building the pilot at the Southwest Research Institute in San Antonio, Texas. For $250,000, the institute would provide Novek a small team of contract workers, a project manager, and, of course, the equipment needed to test his technology. To pay, Novek used all the money he had won from science fairs, and raised the rest through family and friends.
Struggles of an inventor
I met Novek nine months after he started building the plant. It was September 2017 and I was expecting him to be excited about getting to show off his technology at the X-Prize semi-final in October. But he said he had pulled out.
The X-Prize competition asks teams to not just capture CO2, but also convert it into a valuable product. If Novek were to stand a chance of winning, he would have had to partner with another team working on using CO2 to create products like plastic, chemicals, or concrete. But Novek was singularly focused on capture technology.
It’s a valid position. Remarkably, among the many startups I interviewed for this Quartz series on carbon capture, very few were working on making the capture process cheaper. Most were invested in finding ways to make products from CO2. But in the long run, the world will need to capture as much as 6 billion metric tons of carbon dioxide per year, and realistically, only a tiny fraction of that could be converted to useful products. In other words, cheaper capture is likely to be more valuable to society than any useful products that could be made from CO2. Novek gets that.
After pulling out of the X-Prize, Novek has doubled down on his tech. He’s secured funding from an investor to build another pilot plant that will use actual waste gas from a power plant or chemical factory, and capture 1,000 kg of carbon emissions per day. (Novek wouldn’t say who the investor is because of a confidentiality agreement.) He’s also currently applying for a $3 million grant from the US energy department.
There aren’t that many startups working on reducing the cost of carbon capture. There are also few, if any, teenagers in the business. Novek inhabits an unusual world, where the risk of failure is high and the monetary reward not particularly high. But he is living his dream, and that’s a good thing for the world.
Recently, Novek made the tough decision to defer his place at Yale, where he was accepted to study chemical engineering starting this fall. “I miss the social life,” he says. “But that can wait.”
You can sign up to our newsletter for more stories on the challenges and opportunities of low-emissions technology. The reporting was supported by a fellowship from the McGraw Center for Business Journalism at the City University of New York Graduate School of Journalism.