The future of lab-grown meat is one scientific breakthrough away
To grow meat from animal cells, you need three basic things. First, and perhaps most obviously, you need cells. Second, those cells need to be fed, so you’ll also need a nutrient-dense liquid medium. And finally, you need a bioreactor, which is one of the biggest challenges this nascent industry has yet to overcome.


To grow meat from animal cells, you need three basic things. First, and perhaps most obviously, you need cells. Second, those cells need to be fed, so you’ll also need a nutrient-dense liquid medium. And finally, you need a bioreactor, which is one of the biggest challenges this nascent industry has yet to overcome.
Think of a bioreactor as a mechanical cow. Inside a real animal, you have a tissue structure that contains skeletal muscle cells, blood vessels, and intramuscular fat. Most companies right now are working to grow muscle cells, rather than fat.
In a bioreactor, these cells float in the liquid medium, blithely munching away on a man-made concoction of proteins and other nutrients cells need to grow and proliferate into fat and muscle tissue. The bioreactor is supposed to create an environment that can mimic what happens inside a live animal to grow cells. That means, among many things, maintaining a certain temperature, regulating pH levels, and ensuring enough oxygen is moving around the tank.
When you discuss the details of bioreactors with Silicon Valley’s cell-cultured meat startups, one name consistently comes up.
“Have you talked to Jess Kreiger?” asked Mike Selden, co-founder of Finless Foods, which is working to make sustainable, cell-cultured seafood.
Krieger is the co-founder and chief science officer at Artemys Foods, a Bay Area-based cell-cultured meat startup. But before starting her own company, she worked as a research fellow at New Harvest, a cellular agriculture research non-profit that granted her more than $130,000 to study bioreactor design optimization at Kent State University in Ohio.
“The difficulty of growing primary skeletal muscle cells in bioreactors,” Krieger says, “is partially about the bioreactors, but also the cells themselves.”
As Krieger tells it, the art of making meat exists at the intersection of biology, mechanical and chemical engineering, and computer science. No existing bioreactor design accommodates all the needs of cell-cultured meat companies. That’s because each reactor was created for use by medical researchers and pharmaceutical companies. Growing meat at scale will likely require a bioreactor design that doesn’t exist just yet.
Building a better bioreactor for cell-culturing meat hasn’t, until now, been a priority for the industry. Krieger points to California-based PBS Biotech as one company to keep an eye on; it has created a vertical wheel bioreactor that’s gotten some attention among cell-cultured meat scientists. But generally speaking, very little work has been done in this space. One of the bigger manufacturers of these machines, Merck, for instance, tells Quartz that it has not yet started designing bioreactors for the infant industry.
But that’s changing now that the industry feels it has mastered the science, and turns to questions of how to effectively scale the technology.
The main challenge is how to best get the cells to replicate. One thing they like is to grow together in a cluster. This is made possible by many types of connections that naturally occur between cells. One is what scientists call a ‘gap junction,’ a special intercellular fusing that helps cells communicate with each other. Another connection is made possible with adhesive-like protein complexes called desmosomes. These connections help the cells grow together, the tiny building blocks of muscle and fat. In bioreactors, these clusters not only cling to each other, but find additional support by clinging to the walls of the tank or on special platforms at the bottom of some bioreactor models.
Another tricky aspect is to make sure the oxygen and nutrients in the liquid are evenly distributed, which involves incorporating a jet stream inside the bioreactor that is forceful enough to constantly stir the liquid, but not so strong that it disturbs the cells and breaks the clusters.
If the cell-cultured meat industry takes off, bioreactor manufacturers that have long served the medical research field will have a compelling reason to go back to the drawing board and address some of these challenges. “I think that there’s going to be some really incredible and novel design over the next 10 to 20 years,” Krieger says.
The nascent industry is always exchanging ideas about how to build a better bioreactor. But for now, only five basic designs are used in practice—each with pros and cons. Companies can be secretive about what designs they are using, but broadly speaking there are only a few options available from manufacturers.
1. Stirred-tank bioreactors
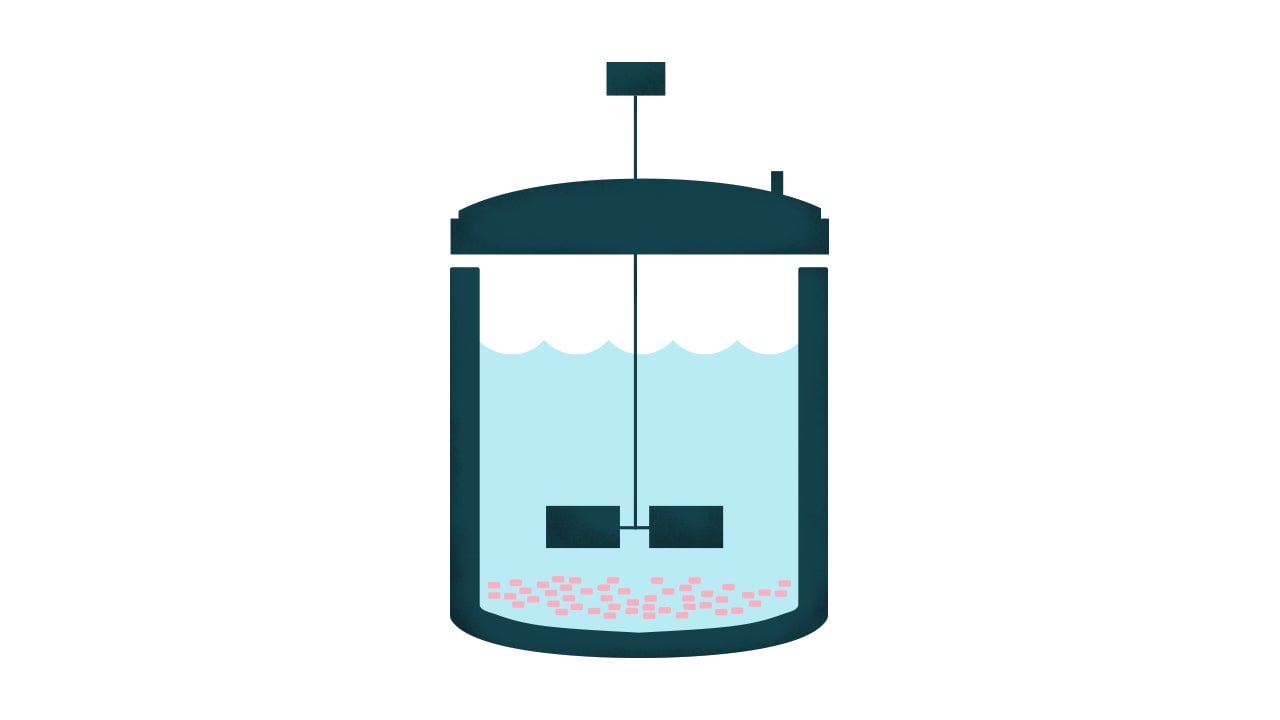
When you step into the laboratory at virtually any cell-cultured meat startup, it’s pretty common to find an Erlenmeyer flask—filled with liquid medium and cells—sitting on top of a scale-like machine that softly gyrates the liquid inside the flask around and around. This system works for growing tiny amounts of meat biomass. But when a company wants to scale up, it becomes complicated.
In a shake flask, there are no internal mechanisms to keep the liquid moving around. That’s fine when the quantities are small as the gyrations do the job. But as companies look to grow more meat, they often opt for a stirred-tank bioreactor. It’s a similar system except that the bioreactor includes a mechanism that moves the liquid around inside the tank.
In medicine, scientists use large-scale, 20,000-liter stirred tank bioreactors for therapeutic protein production. But in that field, scientists are usually working with cells that can just float in suspension, gradually consuming the liquid medium. Mammalian cells, though, like to cling to surfaces where they grow together.
When this system works, a company can achieve good growth pretty quickly. But as scientists look to scale up production, it’s harder to keep the cells happy. As explained in a 2019 review article published in the journal Frontiers in Sustainable Food Systems, the hydrodynamic forces created by the stirring mechanism can detach the cells from the surfaces on which they like to grow, often killing them. A bigger bioreactor needs more fluid, which needs a stronger current to churn the nutrients in the fluid, and those stronger forces can lead to cell death.
2. Packed-bed bioreactors
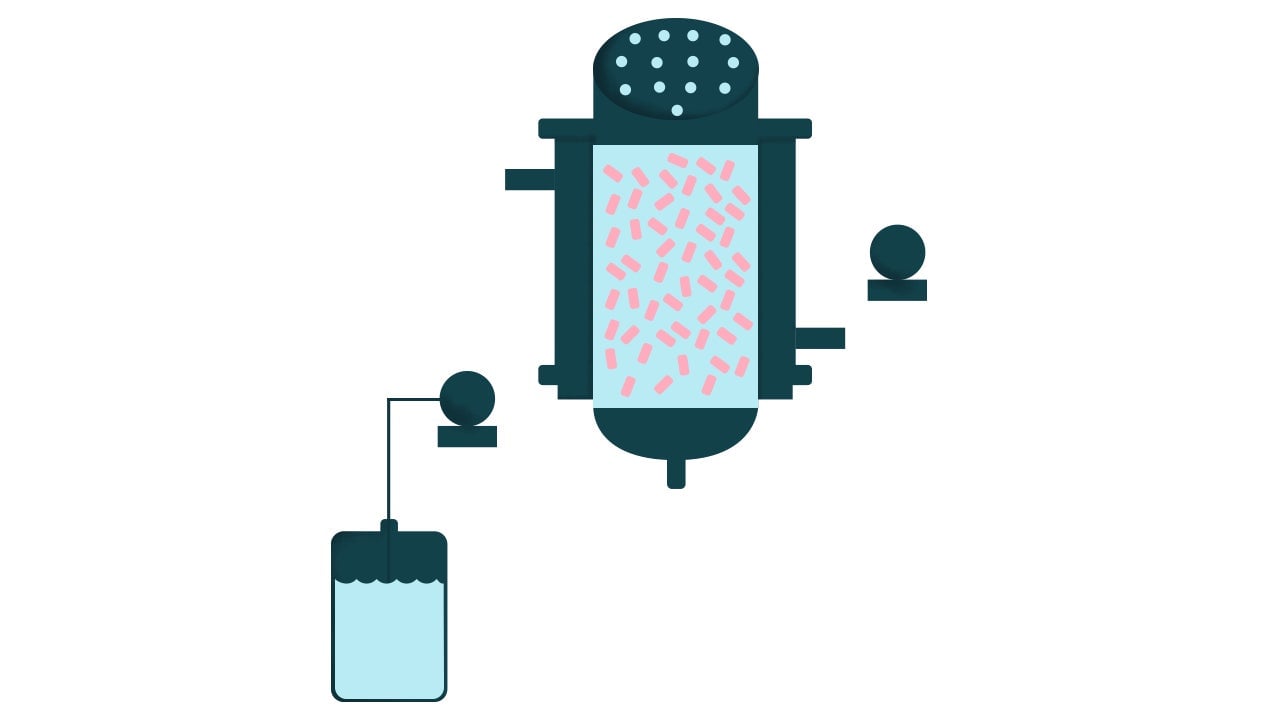
This system has a surface at the bottom of the bioreactor that stays in place and serves as a platform on which the cells can adhere and replicate. Beneath that ‘packed bed’ of cells is a mechanism that creates a jet of fluid that mixes the nutrient-dense medium in the tank, but at a velocity that is sufficiently low to avoid jostling the cells loose from their platform.
One big drawback of this system is that the volume of biomass it can produce is relatively small. The bed of cells has to be harvested, replaced, and the system restarted for each round of growth.
3. Fluidized-bed bioreactors
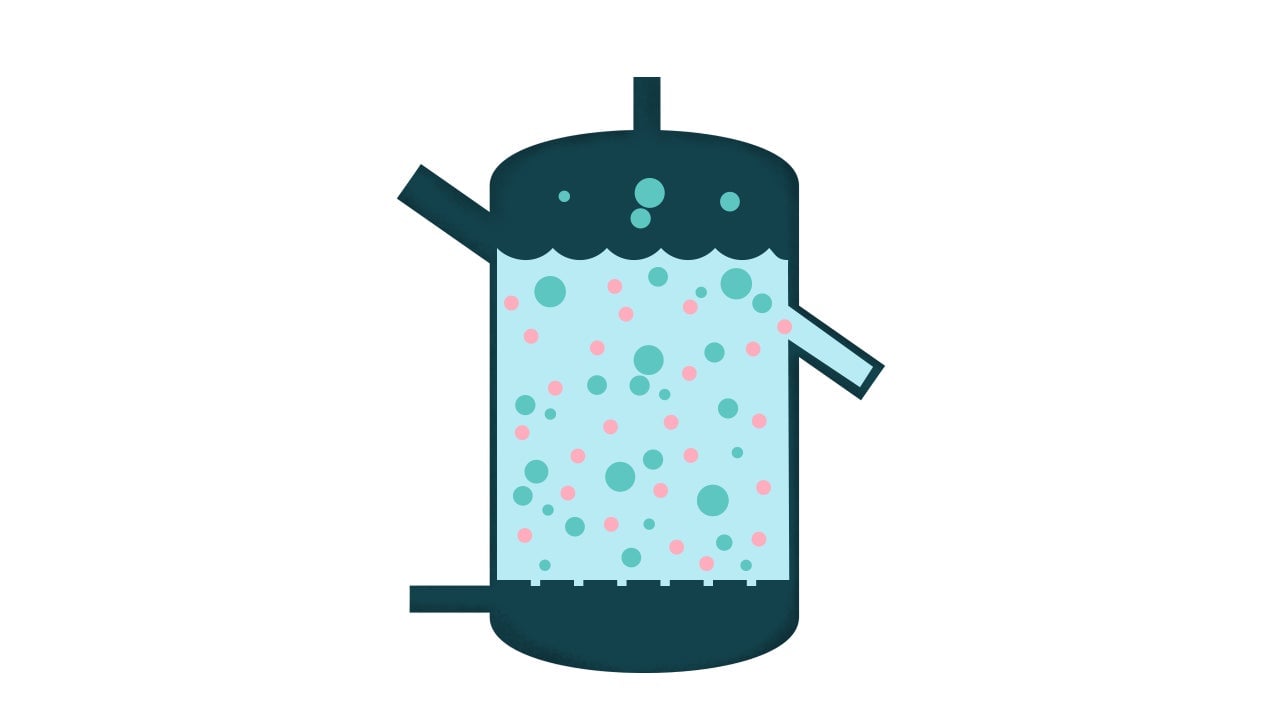
These bioreactors have a similar configuration to the packed-bed varieties in that there is fluid flowing from the bottom. But that fluid has a higher velocity, mixing both the fluid and the cells.
The cells aren’t necessarily growing onto anything, and because of that this design is not optimal for growing mammalian cells. It requires a lot of liquid medium for the cells to float in and feed upon, and it also requires a propulsion system to keep the fluid mixing. Overall, it’s a tenuous system because the cells naturally cling to each other to grow into a mass, and too much mixing will break them apart. Of the different models, this may be the least likely to be used.
4. Hollow-fiber bioreactors
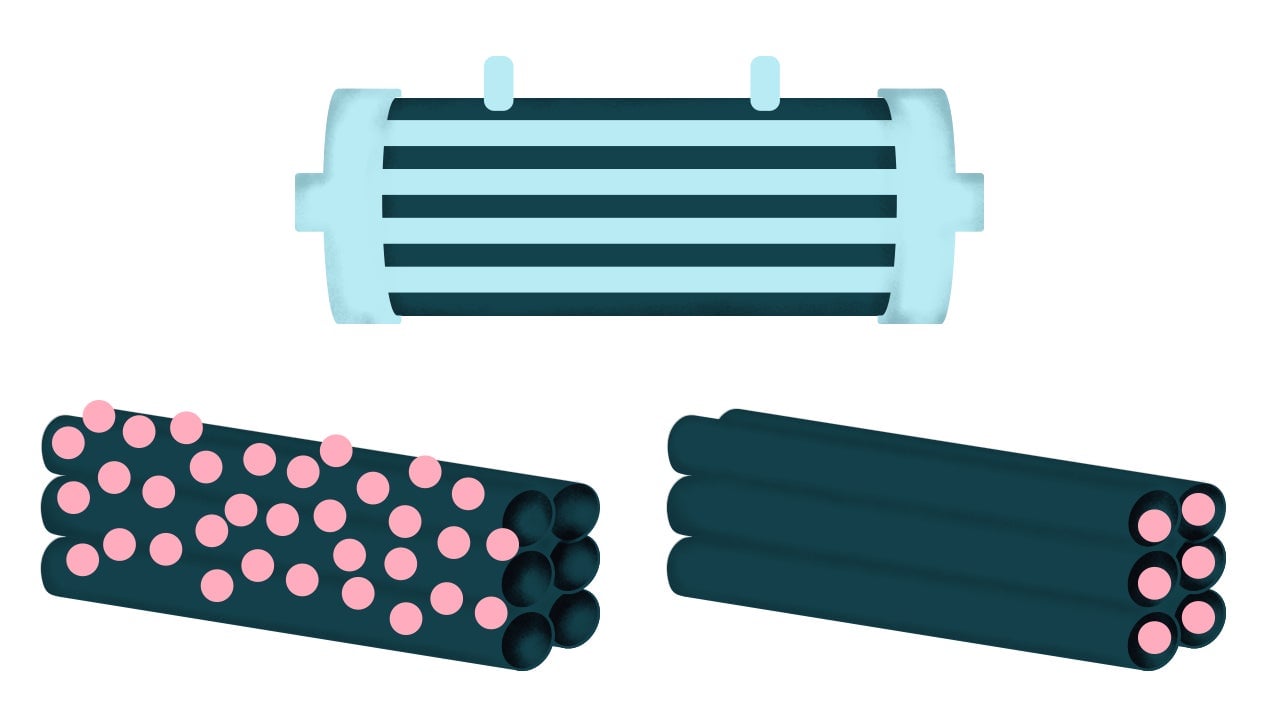
This bioreactor is fundamentally different in how it looks and works. Rather than a tank of liquid medium, imagine a tank filled with a bunch of hollow straws. In this system, the porous straws act like blood vessels. The nutrient-dense liquid is continuously pushed through the straws, feeding cells that soak up the nutrients and grow in the space between the straws.
The best aspect of this bioreactor is that you don’t have to fill a tank with expensive liquid medium. You can use less of it more efficiently, without worrying about jostling cells. The big downside is that it’s pretty tough to harvest the meat biomass that’s created. Pulling apart the straws to collect the cells, cleaning the system, and then restarting the process can be messy and time-consuming.
5. Rotating-wall bioreactors
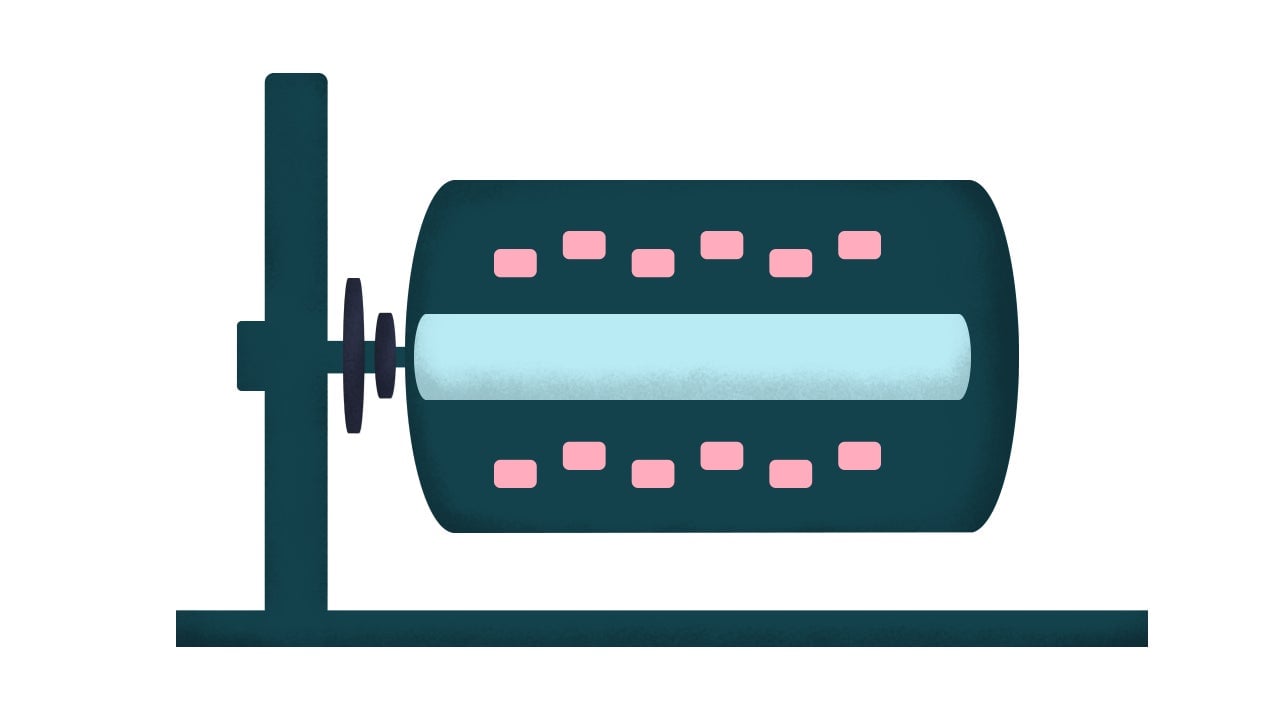
To visualize this bioreactor, Krieger tells me to imagine a soda can turned onto its side. It’s partially filled with nutrient-dense liquid medium, and it spins slowly. Cells grow together along the walls of the bioreactor, feeding as the rotation exposes them to the medium.
It’s not great for growing a lot of cell biomass, but it is considered generally better than the packed-bed bioreactor design.
At the end of the day, building the perfect bioreactor could make or break the scaling of cell-cultured meats. This one engineering problem could be the difference between supplying a single restaurant with meat and supplying an entire city.