There are 13 Covid-19 vaccines in use across the globe just a year after the World Health Organization declared the Covid-19 crisis a pandemic. All of them are shots to the upper arm.
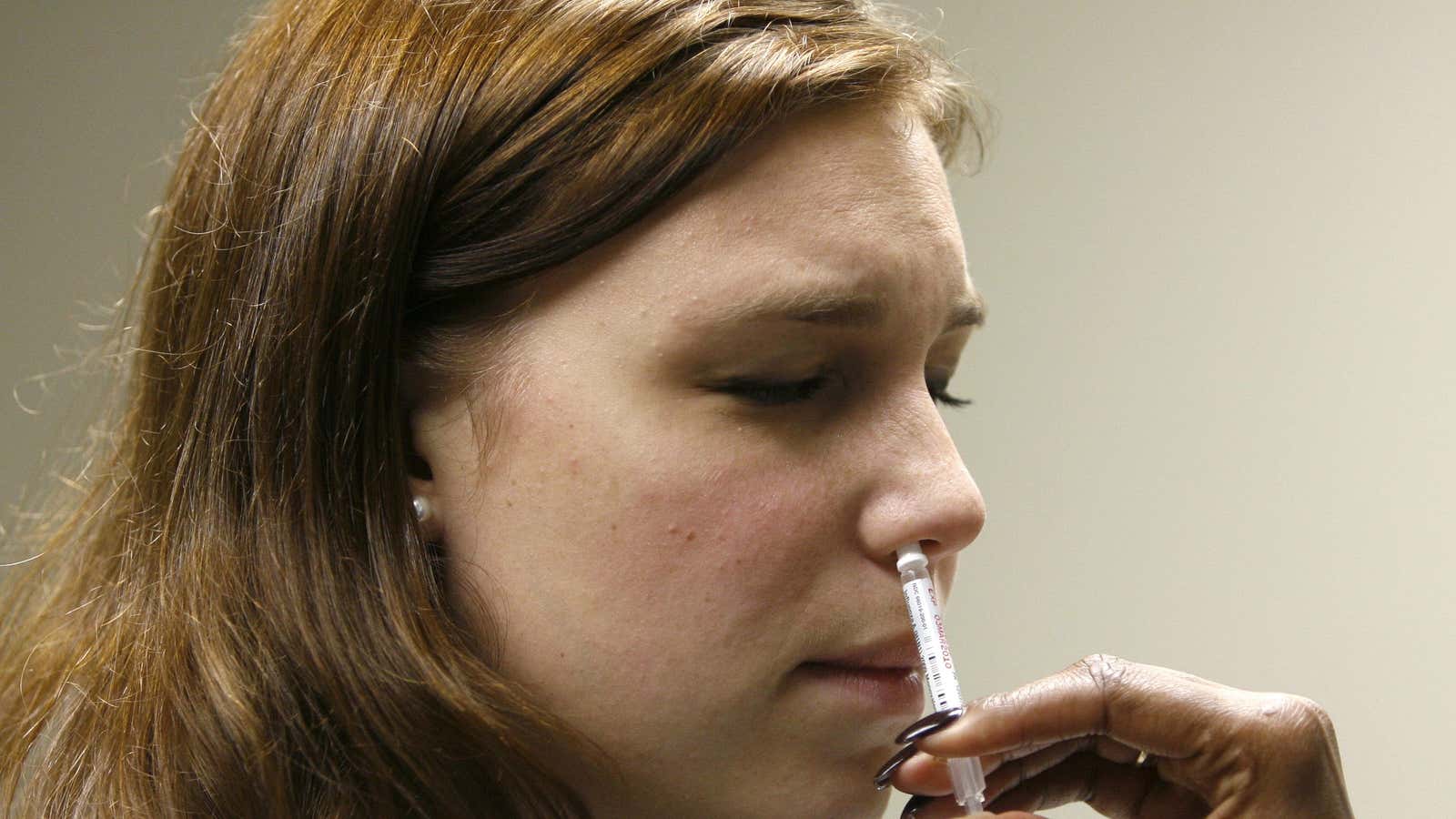
These shots, while a critical tool in ending the pandemic, may not reach everyone. Scientists and drug companies are hustling to find more accessible ways to inoculate people—which includes changing vaccines’ delivery methods. Globally, there are now several Covid-19 vaccine candidates that could be inhaled, instead of injected.
CanSino Biologics, a Chinese drug company announced earlier this month that it received permission from the National Medical Products Administration (the Chinese drug regulatory authority) to start early clinical trials of an inhaled Covid-19 vaccine. In the UK, scientists at the University of Oxford are starting early clinical trials to see if an inhaled version of the AstraZeneca vaccine can produce similar immunity as the injected one, while Codagenix, a biotech startup, is beginning its first round of trials on a novel inhaled Covid-19 vaccine candidate. And in the US, the startups Phage Novo Bio and Precision Virologics—which were spun off from researchers at Rutgers University in New Jersey and Washington University at St. Louis, respectively—have shown through animal trials that their inhaled candidates for Covid-19 vaccines were safe and effective. (Precision Virologics is also sharing the rights of its vaccine with Bharat Biologics in India.)
Inhaled vaccines aren’t new—they exist for other viruses, like flu. But they’re a relatively novel technology arriving on the market in the early 2000s. The Covid-19 pandemic, however, requires the largest mass-vaccination event in history—which means all options need to be on the table for first-round vaccines and any boosters we may need. Inhaled vaccines should be easier to administer, more accessible—and there’s reason to believe they could work better, too.
Inhaled vaccines may be the future
Intramuscular injections aren’t necessarily the best mode of delivery for vaccines—they’re just the most common. Pharmaceutical companies likely chose them for the first vaccine candidates because they’re a tried-and-true method of delivering antigens, tiny molecules that jump start the immune system’s antibody production, and speed was paramount.
But injections aren’t the most accessible form of vaccination for everyone. They often have to be refrigerated or frozen, which can limit the reach of distributions. They require syringes, which are a limited resource, and require a medical professional to extract from vials and deliver into arms.
Right now, even inhaled flu vaccines need to be administered at a healthcare providers’ office. But theoretically, inhaled vaccines can be administered by the recipient themselves, which would alleviate the supply chain bottleneck of syringes. “You could use it like an inhaler, or like Flonase,” an inhaled allergy medicine people can quickly sniff, says Renata Pasqualini, an oncologist at Rutgers University and chief science officer at Phage Novo Bio. “That’s the future.”
Inhaled vaccines could be delivered to people negating the need for a trip to the doctor. “Think about a vaccine you could mail out to people,” says Pasqualini. “People who are homebound or otherwise unable to access clinics could easily gain immunity. It could also be a faster way to distribute booster shots, should we need them.”
Inhaled vaccines may even work better, too. Pasqualini’s animal research for the Phage Novo vaccine suggests an inhaled vaccine generates a stronger immune response than an injected one. It’s unclear why, but it could be with the route of exposure: By going directly into the lungs, the antigens have a chance to trigger elements of the immune system that line the airways. This part of the immune system produces slightly different antibodies than the bloodstream. For a primarily respiratory virus, targeting the mucosal immune system could improve overall immunity—although more research needs to be done to confirm if that’s the case.
So far, all of the inhaled Covid-19 vaccine candidates are either in the animal testing phase, or the first stage of clinical trials testing the safety of these sprays in a very small group of healthy people. Ordinarily, it can take years for vaccines to go from early clinical trials to the market. At the moment, drug regulators have accelerated the authorization process by laying out the exact kinds of data companies need to generate to prove their products are safe and effective. The urgency of vaccinating the globe may keep these high-speed processes in place to get these inoculations into the noses of people everywhere.