To fight deadly disease, scientists are turning genetically female mosquitos into males
Each year, the mosquito that spreads the viral triad of yellow fever, dengue, and chikungunya kills around 56,000 people worldwide, sickening another 100 million. Or to be more precise, the female mosquitoes do. Since mosquitoes suck blood when they need to lay eggs, males aren’t the problem.

Each year, the mosquito that spreads the viral triad of yellow fever, dengue, and chikungunya kills around 56,000 people worldwide, sickening another 100 million. Or to be more precise, the female mosquitoes do. Since mosquitoes suck blood when they need to lay eggs, males aren’t the problem.
Unfortunately, the mosquito species in question likes to live alongside humans, making it freakishly hard to kill with chemicals.
So why not turn females into males? That’s exactly what a technique pioneered by researchers at Virginia Tech is aiming to do, as detailed in a study published in Science Express (paywall). By fiddling with what they believe is a sex-determining gene, the scientists changed the sex organs of two-thirds of genetically female mosquitoes—leaving many with a hodgepodge of male parts.
Finding this genetic switch is, in itself, remarkable. The sex-determining gene—which they named “Nix”—resides in what’s called a genomic black hole, meaning a region of the mosquito’s genome tangled in a thicket of repeating gene sequences. Once they located Nix using computer analysis, the team used a gene-editing tool to turn it off. The majority of the males were born with “feminized” or otherwise impaired reproductive organs. They then injected Nix into embryos; more than two-thirds of females developed some combo of male genitals and testes.
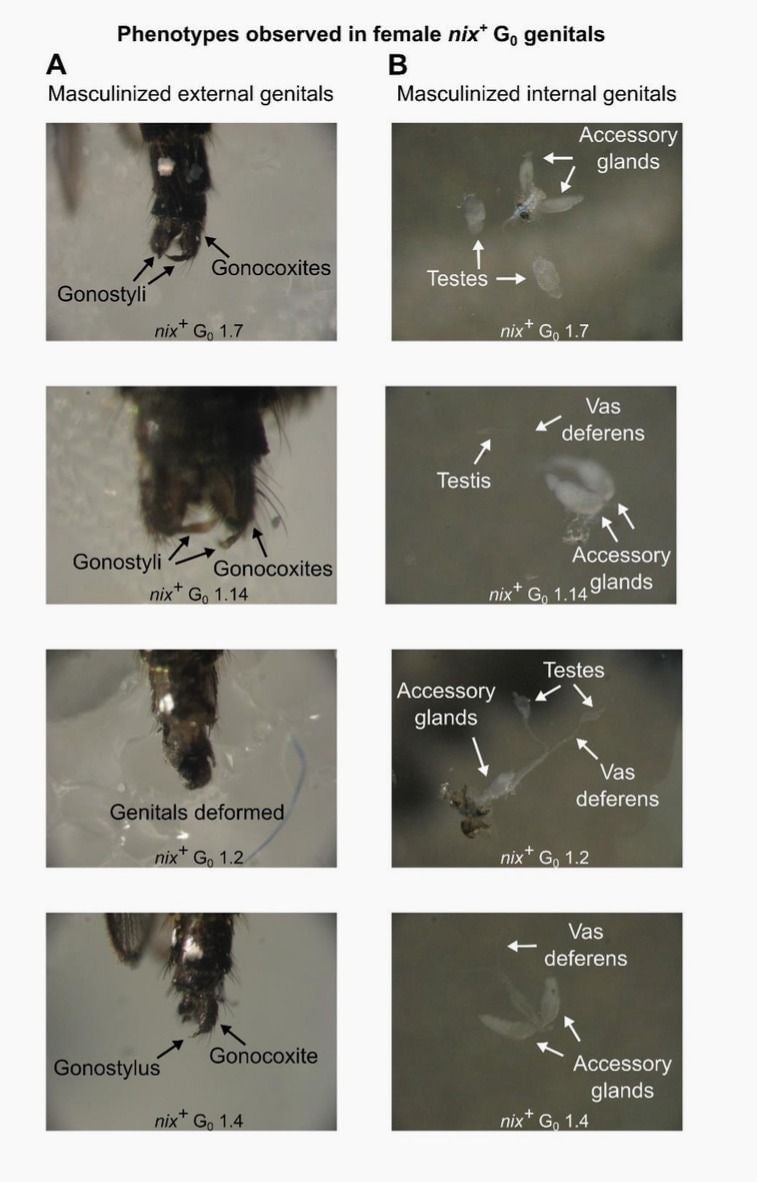
That’s where the exciting potential lies: Without the ability to lay eggs, these females won’t need to bite people (and, obviously, they won’t add to the population of future biting females).
There’s still a ways to go before scientists can start flipping mosquito sex outside the lab, though. One huge challenge is figuring out how to spread Nix to female mosquitoes in the wild.
For a slew of reasons, this could prove tricky. Nix is found only in males—who, in turn, only pass it on to their sons. In the experiment, researchers scrambled the sex organs of females mosquitoes by injecting Nix into embryos one by one—something you obviously can’t do in the wild.
To get around this, the plan is to integrate Nix into the part of the mosquito genome that is inherited by both males and females, says Jake Tu, one of the lead authors of the paper and a biochemistry professor at Virginia Tech. Males carrying this female-affecting Nix gene could then spread it into the wild population.
The next hurdle is raising a big enough transgenic line to make a dent in the wild population, which obviously requires that the treated mosquitoes multiply through reproduction. But if the gene is functioning, the line won’t reproduce in the lab, so growing this line will require a mechanism for turning the Nix gene on and off. Tetracycline is one possibility; the antibiotic has been used to switch off a destructive gene in other transgenic lines.
A broader obstacle—one that makes genetic modification tough in general—lies in the difficulty of creating a consistent, predictable effect by manipulating a single gene within the mosquito’s highly complex genome. The high variation in Nix’s effect on genetic females suggests the gene alone does not appear to determine sex, says Walter Tabachnick, a population geneticist at University of Florida who is unaffiliated with the study. Virginia Tech’s Tu, however, says that injecting the gene into the embryo likely produces more variation in outcome than integrating Nix into the genome would. To stand a chance in fighting dengue and the other viruses, Nix will have to neuter a lot more females than two out of three.
Lead image by the US Department of Agriculture’s Flickr account.