It’s America’s closest thing to a tropical tourism mecca. But Key West’s biggest attraction isn’t the sun-splashed beaches. Its charm lies in its balmy evenings, with their open-air parties steeped in tequila and rum runners, drawing a blend of tourists and locals who pack the pastel-gothic Old Town in search of boozy debauchery.
And they, in turn, attract other revelers. With few screens or doors to stop them, mosquitoes cruise the bare ankles like college kids on a bar crawl. Put their ”bloodmeals” under a microscope, and you’ll find a knot of proteins swaddling a single strand of RNA, better known as dengue. In two epidemics since it reached the Florida Keys in 2009, the virus has sent scores of people to the hospital, racked with the arthritic pains that in days of old earned it the name “breakbone fever.”
Soon, though, the Keys could be facing something much scarier than a bout of achy joints. For centuries, dengue epidemics around the world have been unpleasant, but seldom deadly. However, since a lethal variant called dengue hemorrhagic fever (DHF) emerged in the 1950s, the frequency, intensity, and deadliness of dengue outbreaks has surged. Clinical dengue cases are 50 times as frequent as they were in 1960. Back then, dengue killed 210 a year, on average; in 2007, it claimed 26,000 lives. There is no treatment for dengue, nor any vaccine.
What spawned DHF was a nasty biological quirk—the product of a complex, unforeseen interaction between dengue, the mosquitoes that transmit it, and some three centuries’ worth of globalization. Thanks to this, and to its large influx of tourists, Key West runs an especially high risk that its next dengue outbreak will include DHF. And that could send the Keys’ $1.6 billion tourism industry into a tailspin.
The fear that DHF could put a stop to Key West’s endless party is why the local government is proposing something radical: the first trial of genetically modified mosquitoes on US soil. The insects’ genes are programmed so that their offspring die before reaching adulthood. By continually pumping these tweaked mosquitoes into the Keys, where they will breed with the local mosquitoes to produce autocidal infants, the hope is to ultimately wipe out dengue-carrying insects in the area.
Compelling evidence suggests the technology is safe. It is still new, though, and some worry that these “GM mutants” or “Frankenskeeters” might scramble the food chain, invite in other disease-carrying mosquitoes, or have other unforeseen consequences.
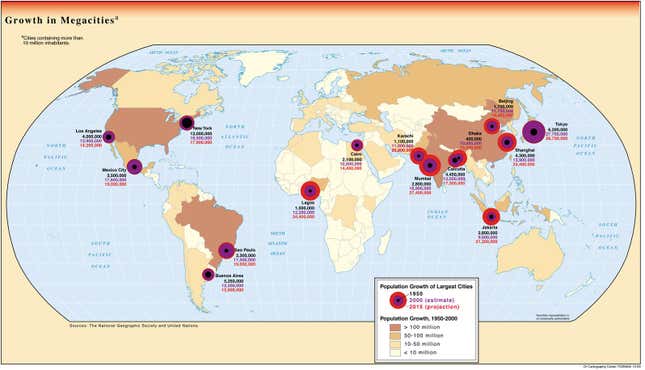
The Florida Keys Mosquito Control District, the government agency tasked with battling the biting insects, awaits approval from the US Food and Drug Administration (FDA) for the trial; a decision could come any day. But what the FDA decides—and how the public reacts—will ultimately influence how other governments combat the disease. Dengue currently infects an estimated 390 million people each year, of whom 96 million develop symptoms, draining an estimated $40 billion from the world economy in health-care spending and lost productivity. Killing dengue-carrying mosquitoes costs governments many billions of dollars more. The virus’s disquietingly swift spread means 3.6 billion people now risk infection in their homes and communities.
This map charts dengue occurrences between 1960 and 2012, using the most comprehensive database of the disease. The data, which were compiled in a project led by Jane Messina, a medical geographer at the University of Oxford, pinpoint each to the narrowest possible location.
This alarming viral conquest is by no means limited to dengue. Viruses most of us had never heard of a decade ago are sweeping whole continents. Take, for example, West Nile, a brain-swelling virus that in 15 years has spread from New York City to every state in the continental US, killing around 20-fold more people each year. Or chikungunya; famed for sickening Hollywood starlet Lindsay Lohan, its more impressive feat is infecting 1.4 million (pdf) in the western hemisphere in just two years—including in Florida (pdf).
Their sudden emergence is thanks to an intricate interplay between the genes of tiny beings and the vast cities and transportation networks that connect our modern world. Dengue’s rise illustrates this most clearly—as do the risks we now must consider as we seek new tools to fight it. How we embrace this technology now will influence not just our odds against dengue—or even the next global mosquito-borne plague—but how we begin reckoning with the ugly risks we’ve already created.
Outbreak in the Keys

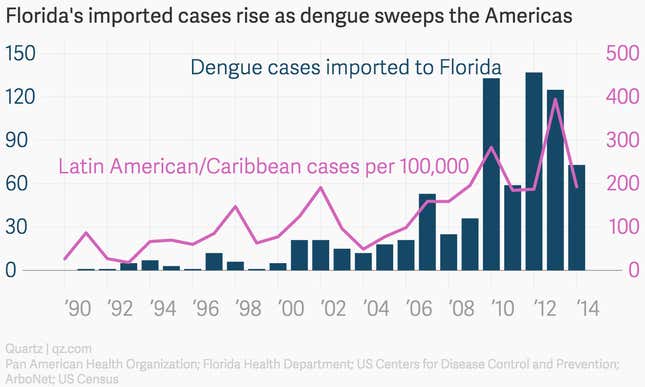
The first sign of Florida’s dengue menace appeared not in Florida, but in upstate New York in early August of 2009. A woman turned up at her local hospital with a fever, eye pain, and a hammering headache—symptoms of dengue. Doctors were puzzled. Scores of American travelers return from abroad with the virus each year, but she had not left the country, and no one had gotten it in the continental US (at least, not outside the Texas-Mexico border area) since 1934.
The woman had just spent a week vacationing in Key West, and it seems certain she contracted dengue there from a mosquito bite. But where did the mosquito that infected Patient Zero pick up the virus in the first place? We can’t know for sure, but Mark Whiteside, medical director at the Monroe County health department, has some guesses.
“What probably happened back in 2009 is someone probably came in [to Key West] from Latin America, stayed at a little guesthouse and sat around with not much on at the pool, maybe didn’t feel too good, but maybe wasn’t that sick. And one of our mosquitoes bit them,” Whiteside told Quartz during an interview in his Old Town Key West office. “[The virus] has to reproduce in the mosquito for a few days, and one thing led to another—it started to transmit it to someone else.”
When it sucked this hypothetical vacationer’s blood, the female aegypti—in mosquitoes, it is only the females who bite—slurped up enough of the virus to itself become infected. The dengue seeped out of its gut, replicated throughout its body, and eventually spread to its salivary glands, a process that can take several days. A few days later, the mosquito encountered someone else—perhaps the woman from New York. Since the thickness of the blood relative to a mosquito’s tiny proboscis makes it rather like trying to drink a frozen milkshake through a straw, the female aegypti would have spat into the tiny wound to dilute the blood, thus infecting this new host with the virus in her saliva.
The Centers for Disease Control (CDC) soon confirmed the bad news: Dengue was spreading in Florida for the first time in 75 years. Key West closed out 2009 with 27 confirmed dengue cases. The next year, in 2010, 63 people there caught the virus.
In both years, said Whiteside, the real number of dengue infections was probably a lot higher. On average, most dengue-infected people don’t fall sick enough to disrupt their lives. Yet they still pass the virus on to mosquitoes that bite them, which then pass it on to other people—so-called “silent transmission.” Subsequent testing found that the 2009 outbreak had infected 5.4% of Key West’s residents—about 1,000 people. No government surveys have been done since then. However, testing done at Key West grocery stores in March 2012 found evidence of “an undetected outbreak in early 2012 of similar or greater magnitude than in 2009.”
Worried about scaring off tourists, local media and some officials downplayed the 2009 outbreak as caused by a ”somewhat tame” disease (paywall). What they largely failed to grasp was that it might not stay tame much longer.
Prelude to a crisis

Four strains of the dengue virus circulate the globe. People who have been exposed to one strain develop antibodies, and thus resistance, to that strain. But they also develop a higher risk of contracting the lethal variant, dengue hemorrhagic fever, should they later catch one of the other three strains. What happens is that, instead of attacking the new dengue invader, the antibodies from the first infection bind to it. Effectively, they turn into weapons for the virus, allowing it to hijack the immune system’s main killer cells, invade the organs and copy themselves faster—a process that will eventually sicken the human host more.
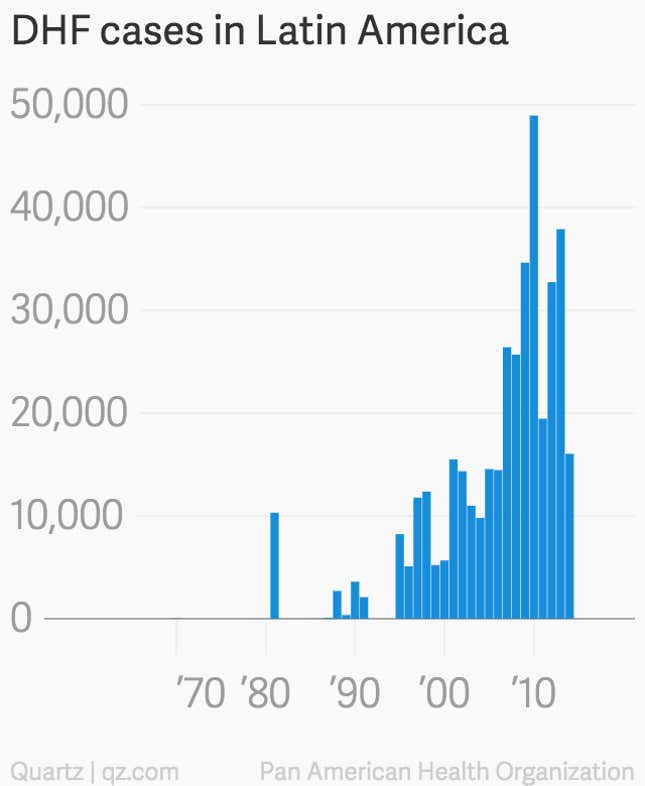
DHF is as nasty as its name suggests. It causes the circulatory system to shut down (pdf, p.15), leading to internal bleeding. If it’s treated early, plasma and fluid transfusions can save patients. But in severe cases, as blood flow slows and clotting fails, shock sets in, followed eventually by organ failure and death. Anywhere from 1% to 5% of those who get DHF die, and in areas with shoddy health care, the death toll can reach 30%. Multi-strain epidemics now abound in Latin America and the Caribbean—and as they’ve increased, so have the number of people turning up in Florida hospitals with dengue.
Even today, dengue is commonly classified as a “neglected disease,” even though more than half of the world’s population lives in areas where the virus is endemic. One reason is simply that it’s not very deadly. In an average year, only about 22,000 people die of the estimated 390 million who are infected. Compare that with the 584,000 that malaria kills, out of 198 million infected.
But the reason it’s so easily ignored is exactly what makes it so insidious. Three-quarters of the 390 million barely notice any symptoms; another 96 million experience a week or two of fevers and debilitating aches. People with less severe symptoms can walk around, giving mosquitos more opportunities to pick up, and then spread, the virus. This means that when multiple dengue strains reach an area, it’s easy for people who’ve already had one strain to contract one of the others, making them more susceptible to DHF.
In those patients, the rapid replication of the virus also makes it spread more easily to a biting mosquito and other human hosts, increasing the chance of DHF outbreaks. These can be much deadlier than the global total of dengue cases suggests. Most of the 22,000 worldwide deaths are due to DHF, so as DHF appears in more places, the total dengue death toll could start to go up very quickly. Before 1970, only nine countries had DHF epidemics; now more than a hundred do. Each year, 500,000 are hospitalized with DHF.
Hunting a hidden killer

In early April, I took a trip to Key West to speak with the Florida Keys Mosquito Control District (FKMCD). It is the Top Gun of mosquito-killing, an agency that many in the know call the world’s best. Prior to 2009, FKMCD’s reputation was based on its knack for massacring ”nuisance mosquitoes” that breed in Keys salt marshes. “Most of the mosquito control was done using DC-3 airplanes literally spraying square miles every night, blanketing the area,” said Michael Doyle, the agency’s director since 2011. ”It’s been done this way since the ’40s.”
Outfitted thus, you’d think FKMCD would have handily beat back Aedes aegypti, the mosquito that spreads dengue. ”All of a sudden we had this mosquito we didn’t really pay any attention to before,” Doyle told Quartz. “So we started carpet-bombing the area [with insecticides].”
The noxious blitzkrieg didn’t dent aegypti numbers at all.
As it happens, the small, black-and-white-striped insect is one of the planet’s toughest to kill. That’s because aegypti tend to live indoors, preferring the shadowy recesses of closets, curtains, and the undersides of beds to stagnant salt-marsh muck. They breed in small pools of clean water, such as can be found in garbage cans, bird ponds, boats and jet skis. This cozy cohabitation with humans puts them well beyond the reach of insecticides sprayed from airplanes. Moreover, their peculiar skittishness means a single aegypti will make multiple sorties on a human before finally feeding successfully. Each attempt ups the likelihood of spreading the virus, since the mosquito injects her saliva each time, even if she doesn’t manage to feed.
The FKMCD shifted from its traditional tactics to conduct surgical strikes on aegypti, dispatching 20 inspectors door to door in Key West to dump out containers where the species might breed. Even that only cut aegypti numbers in half, said Doyle.
That’s not nearly enough to stop an epidemic. In their 2010 sweep, the agency’s inspectors found aegypti breeding in or around 12% of Key West homes. While scientists disagree on the level necessary to prevent dengue epidemics, the FKMCD said in 2010 the Keys’ threshold is 2%.
Around that time, the agency decided to try a novel approach: genetically modified mosquitoes.
The killing gene

The logic is appealing. Humans have a hard time finding aegypti. But other aegypti obviously don’t. So use the aegypti to kill each other. That’s the premise behind a technology pioneered by a group at the University of Oxford that spun off into a company called Oxitec.
Oxitec has tweaked the genes of aegypti with proteins snipped from a random assortment of things—E. coli, the herpes virus, and cabbage, to name a few—giving them a protein that kills their offspring before they reach adulthood. The deadly protein stays in “off” mode as long as the GM mosquitoes are fed tetracycline, which is how Oxitec keeps growing the line in its labs. Once the male GM mosquitoes are released into the wild, they mate with wild females. Deprived of tetracycline, the protein lays waste to their offspring’s tiny bodies.
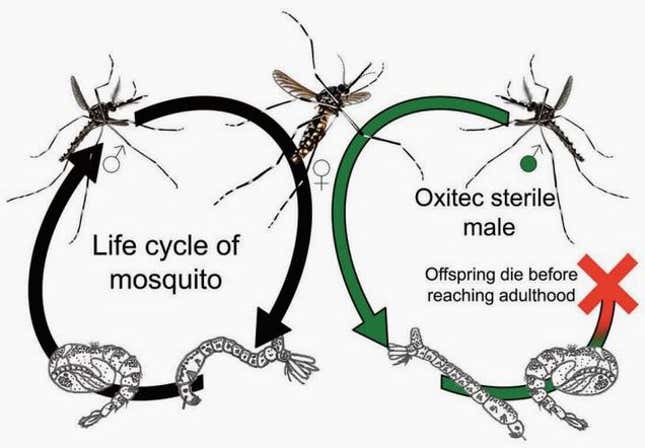
Trials in the Cayman Islands and Brazil have diminished aegypti populations by 90% or more. Doyle and his agency are hoping for a 95% reduction in Key West if the FDA approves the 22-month trial. Oxitec will provide the mosquitoes—potentially many tens of millions—for free. If the trial is a success, the FKMCD will have to pay to keep using the mosquitoes, but Doyle said he doesn’t anticipate the agency spending more than it does on current control methods.
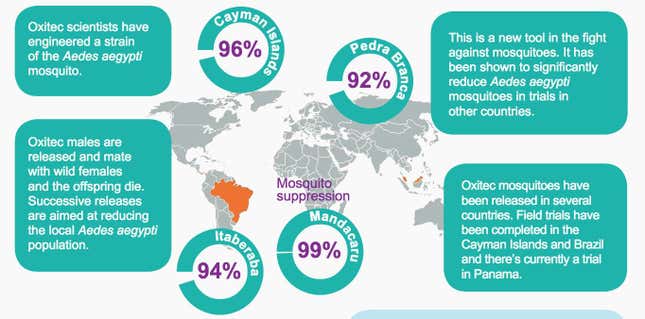
Even if they’re eliminated, aegypti will, of course, keep coming into Key West, stowed away on Panamanian shrimp boats and in Home Depot plant trivets. So once the Florida Keys have signed on to Oxitec’s plan, they’ll likely have to keep buying a set number of the insects indefinitely—a sort of mosquito subscription service.
Unintended consequences
What the FDA will have to decide is whether releasing GM mosquitoes could backfire in some way. That decision could come tomorrow, or in three years (a spokeswoman says the FDA can’t say when it will complete its review). If the FDA okays the trial, opposition might still derail things. “The fact of the matter is, there’s a small but very vocal group in Key West and elsewhere that thinks it’s a horrible, terrible plot,” said Florida health department’s Whiteside. “So I think there’s going to be continued resistance to it.”
Those unsettled by the trial tend to cite ”unforeseen consequences” (usually followed by an allusion to Michael Crichton’s Jurassic Park)—a frustratingly vague rhetorical device. ”From a public-health point of view, we’re talking about risk that is palpably small in the face of trying to control an epidemic that could kill people and certainly cause many, many people to be very sick,” said Walter Tabachnick, an expert on aegypti population genetics at the University of Florida.
Nonetheless, it’s true that humans have a lousy track record at seeing the long-term environmental consequences of our behavior. The very threat that Key West is now trying to stamp out exists because we ourselves unwittingly spread aegypti—and therefore dengue, and its deadly variant DHF—far and wide around the globe.
To understand those unintended consequences, we need to go back into the annals of how the disease emerged—and how aegypti got to Key West from its ancestral home more than 4,000 miles away.
Out of Africa
For countless eons, Aedes aegypti zipped around the African jungle canopy, eating, mating, and doing little else. The mosquitoes ate mostly fruit juices and plant nectars. But when a female was preparing to breed, she would touch down on a monkey or another jungle creature and suck up a bloodmeal to nourish the few hundred eggs she was about to lay.
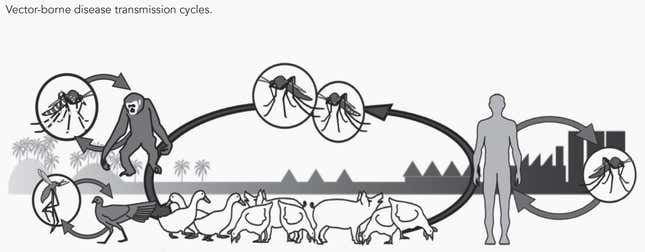
Then, at some point in the mid-1600s, maverick aegypti stowed away on European ships docked in West African ports, set to traffic African slaves to the New World.
In the ghastly cargo holds of slave ships, the female aegypti found that human blood was a more than acceptable substitute for monkey blood. The passengers—particularly the captive slaves—provided a steady supply of it, allowing the mosquitoes to lay their eggs. Barrels filled with rainwater to drink made more reliable breeding sites than the hollows of the trees they were used to.
Even back then, viruses that exploited aegypti‘s fondness for humans often thrived too. When these ships anchored in the Americas, aegypti had spent months swapping these viruses with the people destined for bondage on the booming sugar plantations, as well as the sailors who enslaved them. The dense settlements of colonial New World ports and plantations were fertile ground for both the mosquitoes and their viruses.
Dengue was barely in the picture back then. Aegypti‘s far more notorious pairing was with yellow fever. The two went way back, having met in West African rainforests many centuries before and, among other things, mowing down European imperialists who pushed too far inland from African coasts. Unleashed on the New World, the familiar pair laid waste to coastal cities up and down the Atlantic for 250 years.
Until the mid-1600s, dengue lived only in Asia. It jumped into humans from Malaysian rainforests via Aedes albopictus, the Asian tiger mosquito. However, albopictus‘s taste for animal blood and preference for the outdoors made it less adept at spreading the virus. The beginnings of globalization introduced dengue to aegypti for the first time in the Caribbean. Their rapport was soon clear. By the early 1800s, dengue epidemics commonly blighted port cities all over the Americas, hitting every 30 or 40 years.
Still, hardly anyone ever died. So what changed to make dengue so deadly in the 20th century?
The making of a modern menace
Evolution is part of the reason. Though they share a common viral ancestor, each of dengue’s four strains spilled independently out of Malaysia’s forests and into villages and rural communities around southern Asia. In the last 60 or so years, however, they have been evolving—so rapidly, in fact, that each strain now has distinct geographic sub-strains of varying virulence.
Some of this genetic change has been random. But much of it is not. Research shows that when a new dengue strain hits virgin soil, it branches into new lineages at an exponential rate (pdf, p.8; paywall). As it does, the epidemic’s pace and severity often rise too.
But what enabled this evolution were creations of human beings: specifically wars, cities, and planes.
Until the 1960s, new strains seldom made it to virgin soil, largely because most travel took place by boat. Someone who picked up dengue just before boarding a steamer from Mumbai would arrive in Guangzhou about 17 days later. Since the virus’ incubation period is at most two weeks, it couldn’t spread. Up until about World War II, the stately pace of travel kept the rate of dengue transmission relatively constrained. But then the tempo picked up.
“What I call the 20th-century pandemic of dengue started in Asia after World War II, when both armies spread throughout the region,” says Duane Gubler, an epidemiologist at Duke-NUS Graduate Medical School in Singapore and one of the world’s foremost dengue experts. As the Allies and Axis moved huge phalanxes of troops throughout the Asian and Pacific theaters, the mosquito and the virus tagged along—aegypti in the cargo holds and tires of transport vehicles, the virus in the bodies of soldiers. Tight living quarters and the tropical rains made it easy for aegypti to breed, and for the virus to replicate. All over the region, these movements brought the four dengue strains into areas that previously only had one. By the time the Paris Peace Treaties were signed, aegypti had colonized Asia.
The postwar boom brought more people to developing-world metropolises. Yet spending on infrastructure failed to keep up. As the populations exploded, so did their urban slums. Tin roofs, tires, and trash created new breeding grounds for aegypti. Crowding let them whisk the virus from person to person quickly. Perhaps most importantly, filthy water and the lack of sewage meant families had to set aside clean water in buckets, the mosquito’s ideal breeding ground.
Since dengue so often spreads in secret, air travel constantly replenishes the supply of unwittingly infected people. Dengue’s antibody trick—the fact that antibodies against one strain can become weapons for another—gives the virus far more opportunities to copy itself, which ups the chances of a mutation that will help it spread even more rapidly. The Jet Age has boosted those odds, making it far easier for humans to carry an emerging dengue sub-strain to virgin soil. And once the virus infects enough people to become endemic, steadily bigger and deadlier DHF epidemics soon follow.
The first solid evidence of this came in 1953, when an epidemic of dengue struck Manila. Unlike in previous outbreaks, the symptoms this time included internal bleeding, shock, and death. Two more big outbreaks hit Bangkok in 1956 and 1958. Nearly 2,300 were sickened, most of them children; 240 people died. Microbiologists soon discovered the unsettling fact that most of those with DHF had already been exposed to another strain of dengue at some point in the not-so-distant past. Within a decade, dengue epidemics had become the top killer of children in Southeast Asia.
At first, Latin American and Caribbean cities were spared. Starting in Cuba and Panama in the 1900s—and, elsewhere in the Americas, in the 1940s—governments had launched a war on aegypti to fight the far more serious scourge of yellow fever. By 1962, military-style campaigns and the widespread use of the highly effective insecticide DDT had made 18 Latin American countries and a slew of Caribbean islands officially aegypti-free.
Dengue, too, disappeared—but only for a while. As the region’s governments scrapped mosquito surveillance and shuttered control programs, aegypti was stealthily returning, slipping into ports on cargo ships and in airplane holds. The virus came back too—and this time, not one single strain. Now multi-strain epidemics ravage Latin America. And as Florida’s outbreaks since 2009 attest, it’s been creeping steadily north.
It is this kind of complexity—the myriad unpredictable interactions of biology and human society—that makes people uneasy about Oxitec’s trial of genetically modified mosquitoes in Key West. There are two main sources for the unease. The first is evidence that simply wiping out the Aedes aegypti population may not in itself wipe out dengue. The second is a small but not negligible risk that the concerted genetic attack on aegypti might, through the devious processes of evolution, cause either them or the dengue viruses to become even more implacable foes.
Singapore: A cautionary tale

Oxitec’s official objective is to cut aegypti numbers. By that measure, its Brazil and Cayman Islands trials, which killed 90% of the mosquitoes, have proven successful; the FKMCD is hoping for a 95% reduction in Key West. But Singapore’s bleak experience in fighting dengue suggests that killing mosquitoes alone is not always enough.
Dengue epidemics first hit the island state in the 1960s. The government launched an all-out war on aegypti breeding sites starting in 1967. Over time, it slashed the proportion of premises where aegypti were found to be breeding from 16% to less than 1%.
And unlike its counterparts in Latin America and the Caribbean, Singapore remained vigilant. With the exception of 1973 and 1978, Singapore’s reported dengue cases fell to fewer than 15 per 100,000 residents.
However, as fewer and fewer people were exposed to dengue, fewer and fewer had antibodies. As a result, Singaporeans lost their “herd immunity,” which is when enough people have antibodies to make it nearly impossible for a virus to spread. (A loss of herd immunity to measles in some places is the worrying result of the recent anti-vaccination movement in the US.)
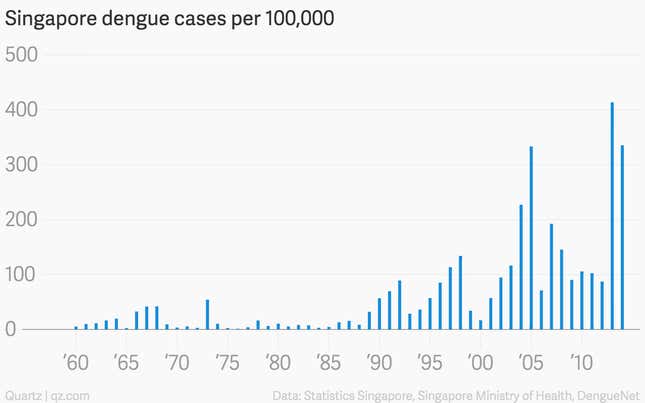
“It was exactly during the time that Singapore’s herd immunity dropped rather low, in the 1970s and 1980s, that the re-emergence of dengue in Southeast Asia really took off,” said Duane Gubler. On top of that, the country was rapidly becoming a regional hub for shipping, finance, and tourism.
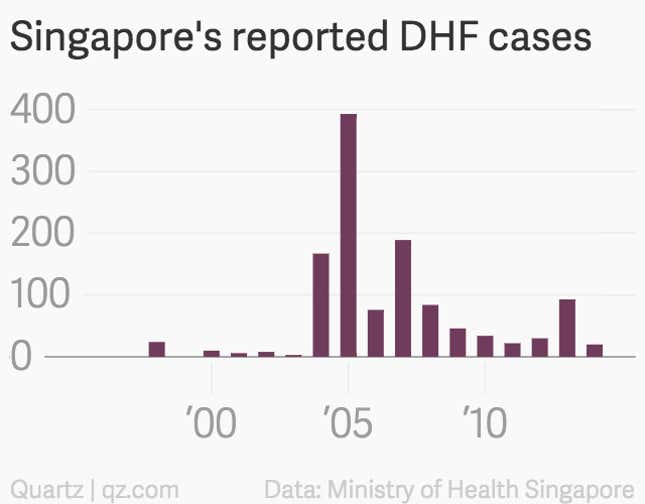
In the late 1980s, the dengue epidemic cycle stirred anew. By now, despite keeping aegypti numbers consistently at the levels they were at in the 1970s, Singapore has 20 times as many dengue cases a year as it did then. Singapore’s problems included a constant influx of travelers, low herd immunity, and, in cars and cargo holds, a small but sure influx of emigrant aegypti.
Key West shares all of these features.
What if the killer mosquitoes don’t kill?

The persistent failure of even the best mosquito-control strategies is why genetic engineering may be a vital weapon in the battle against mosquito-borne epidemics. The thorny challenge is sorting out which of its inevitable risks we choose to acknowledge, which of these we measure, and how much uncertainty we can stomach. The US’s decision will help shape those precedents.
One particular series of risks illustrates this thorniness well: what might happen with the small proportion of Key West’s aegypti population that are expected to survive the onslaught.
The autocidal gene in Oxitec’s GM mosquitoes doesn’t in fact work perfectly. Despite having it, about one in 20 of the offspring of wild females and GM males survive. This doesn’t really matter, said Chris Creese, Oxitec communications manager, because those offspring will still pass the gene on to their offspring, causing 95% of them to die; over a half-dozen generations, the population should fall to zero. That 95% death rate is consistent over 150 generations, said Creese. “In fact, data from field trials show that this die-out happens even faster,” she added, though those data haven’t yet been published.
What’s not clear is why the autocidal gene fails to do its job 5% of the time. Without knowing why, it’s hard to be sure that it will keep on doing it consistently, said Tabachnick, of the University of Florida. That means there’s potentially a risk that, once the mosquitoes are out of the lab and spawning in Key West, the 5% survival rate might somehow rise.
So what if it does? The hypothetical worry here concerns what’s called “vector competence,” a slew of factors that determine how readily the mosquito picks up the virus and passes it on to a new host, as well as how quickly the mosquito’s body allows the virus to copy itself. The spread of the disease depends not just on how few aegypti there are. What also matters is how good those aegypti that remain are at spreading the disease, and whether they pass those traits on to future aegypti generations.
Scientists have found that these factors of vector competence vary a lot by country, region, city, and even district. These differences have been documented in Chiang Mai, Australia, Mexico, Vietnam, Bolivia, Texas, Kenya, and West Africa, among other places.
The line of GM aegypti destined for Key West originally come from mosquitoes collected in Mexico and taken back to the UK, where Oxitec inserted two genes: the autocidal gene and one giving the insect a fluorescent marker (to make it easier to track). The rest of the GM mosquitoes’ genes are still Mexican—and therefore different from those of Key West aegypti.
In theory, then, these genes might include some that, unbeknownst to anyone, gives aegypti a greater vector competence. And if so, the Oxitec mosquitoes would be spreading those genes to whatever percentage of Key West mosquitoes do survive the autocidal onslaught, making them better at spreading dengue. That possibility is very remote, said Tabachnick, but still enough that the ideal would be to breed GM mosquitoes based on local Key West aegypti rather than on another population.
Oxitec’s Creese dismissed this risk. “There is no evidence for genetic variation in mosquitoes causing an increase in viral transmission,” she said. “There is a body of scientific evidence that shows that Aedes aegypti everywhere is effectively the same, except in Africa where it originated. There is genetic variability but it is effectively the same population and they are interbreeding.”
Janine Ramsey, a scientist and professor at Mexico’s National Institute for Public Health (INSP in Spanish), disputed this. While the mosquitoes’ genes themselves may be similar, she said, there is tremendous variation in which genes produce a given effect. The genes that give a mosquito insecticide resistance in one part of the globe, for instance, might not be the same ones that do it in another. Complicating things further, these effects also seem to depend on the particular genes of the virus the mosquito is carrying (paywall).
Tabachnick concurred. ”Some populations of aegypti are better vectors than others. The cause of these differences is simply unknown though it is accepted that the genetic differences between populations does play a role,” he said. “To claim that aegypti everywhere is the same is naive and dangerous, and precisely opposite to all the information we have about this and other species.”
Still another way the GM mosquitoes might pass on their lineage, despite being designed not to, is suggested by a previous Oxitec trial that didn’t work.
In that trial, conducted in Mexico by a consortium of the US’s Foundation for the National Institutes of Health (FNIH), the method was a little different. Rather than killing their offspring, the modified gene was supposed to scramble the muscles of female mosquitoes, making them unable to fly—a big problem for them, since aegypti mate mid-air. It wasn’t supposed to have any effect on males.
However, during an experiment, one of the research partners found that some of the GM mosquitoes only had one copy of the gene rather than the two needed to pass on the trait consistently—meaning half of their female offspring could fly, and mate. The GM mosquito line was likely contaminated during an earlier experiment in Colorado; at some point, a wild mosquito probably sneaked into the GM mosquito insectary. The line returned to Oxitec in the UK before shipping to Mexico, said Luca Facchinelli, a medical entomologist at the University of Perugia, who managed the field site.
“The maintenance of the [GM mosquito] lines and the exchange of the lines between the members of a consortium like this is crucial,” Facchinelli told Quartz. It showed how a slip-up could, in principle, cause imported mosquitoes’ genes to spread through a local population, with unpredictable consequences.
A problem of evaluation

For all these concerns, the risk that Oxitec’s GM mosquitoes might help the spread of dengue in the Keys rather than hinder it is probably minute; the risk of DHF hitting Key West is much, much greater. Trying the Oxitec approach makes sense.
But the controversy shows that what’s at stake here is not only whether to use GM technologies, but also how to measure their effects. Oxitec argues that its method should be evaluated solely on its ability to reduce aegypti numbers, and held to no more regulatory scrutiny than that trained on other “vector-control” approaches, like insecticides or sticky traps. Yet as the experience of Singapore and other places shows, a singular focus on killing mosquitoes has failed to quell the disease, and has sometimes even made it worse. Sweeping changes to a mosquito population can alter its interactions with the virus and human hosts in incredibly complex ways.
This uncertainty means there’s a case for monitoring the Oxitec trial in other ways: studying, say, the spread of Mexican genes in Key West’s aegypti population, or whether the release changes the local population’s vector competence for dengue or chikungunya—a painful virus that aegypti also carries and which is now transmitted locally in Florida.
The trial in Key West could also shape precedents for the use of GM technology more generally, and for tailoring public-health strategies to the deepening complexity of the modern world. Standards for this are already being set. Since 2010, Oxitec has released the mosquitoes in populated areas of the Caymans, Brazil, and Panama, as well as in an uninhabited patch of Malaysia. The company is currently in talks with the Indian government about a trial release. In all these cases, the measure of success is the fall in aegypti numbers. After Oxitec’s success reducing them in Brazil—where the dengue situation is so severe the country sometimes calls in the army to educate people on prevention—the Brazilian government last year cleared Oxitec to release the GM line throughout the country. The company’s first project since winning that approval began just last week in Piracicaba, in São Paulo state.
But the US could be the first developed country to okay a trial release. FDA approval could be a big coup, said Hadyn Parry, CEO of Oxitec, since the US regulatory system is often seen as the international “gold standard.” The protocols and precedents the FDA sets for how GM technology is used and evaluated will therefore have global importance.
The GM future dawns
Whether or not the FDA approves the Key West trial, the appeal of GM mosquito control is unlikely to go away, given how desperate many countries are getting. Dengue costs Latin America around $2.1 billion a year (measured in disability-adjusted life years), on average, making it more expensive than that of any other viral illness. Southeast Asia’s annual dengue burden averages $950 million, more than upper respiratory infections or hepatitis B. And that’s not counting mosquito-control operations.
No country feels this more keenly than Brazil, with three-quarters of the Western hemisphere’s reported dengue cases. The three-strain epidemic raging there has sickened more than 465,000 and killed 132 (pdf) in the first four months of 2015 alone. Its battle against mosquitoes costs Brazil an estimated $1.2 billion annually.
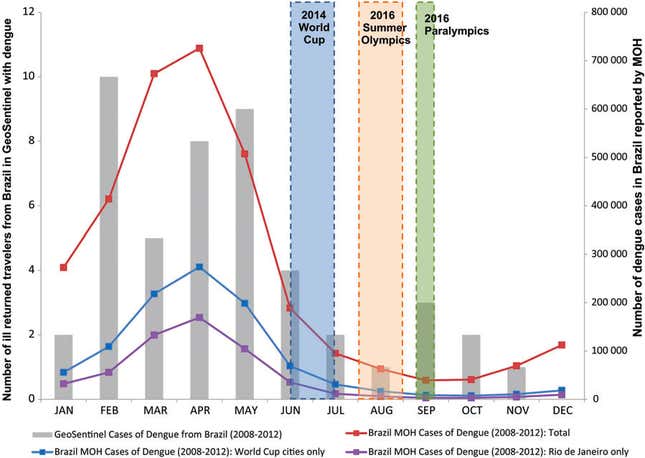
Small wonder, therefore, that Brazil is at the vanguard of experimenting with genetic modification and other molecular-level technologies. With the 2016 Olympics looming, pressure is mounting. Fortunately, the games start during the low season of dengue transmission. Brazil got through the World Cup, which fell slightly higher in the season than the Olympics will, without any major problems. Still, the prospect that an epidemic might sicken athletes and visitors is a big worry.
Dengue also exacts an economic toll on individuals, particularly in countries with shoddy health infrastructure. Puerto Rico spends an average of $46.5 million a year on dengue, half of which is shouldered by households (the government and insurers cover around a quarter apiece). The poorer the country, the more that burden tends to shift to families; Cambodian households, for instance, pay nearly 80% of overall costs.
Since dengue affects tropical countries, tourism revenue is inevitably on the line as well. Once word gets out that a dengue outbreak is underway, tourists tend to quail. A major epidemic in Thailand could trigger losses of as much as $363 million in tourism revenue (pdf), according to a 2009 study.
Most of these countries are much poorer and much sicker than Key West. Every investment comes with a big opportunity cost—money that might instead be spent on things like basic health care and sanitation, which might themselves help combat diseases like dengue. That makes the lack of evidence that Oxitec’s mosquitoes can reduce dengue transmission more worrying.
“In the end, nobody can take away from the fact that [genetic modification in Oxitec’s GM line] is good science,” said INSP’s Ramsey. “But it’s not there today and the push to get governments to spend massive quantities of money on something that hasn’t been properly evaluated from the beginning—and not on essential primary preventive interventions—that’s unethical.”
In Florida, though, Oxitec’s killer mosquitoes are the only feasible quick fix for preventing another dengue epidemic, said Michael Doyle, the FKMCD head. ”In a position like mine, where I’m concerned about people getting dengue this summer or next summer, the thought of saying, ‘Well we’re not going to try anything for a decade because we want to do a long [study] with lots of modeling that might not prove anything’—that’s really distressing.”
The rainy season, which lasts from May to November, is now descending on the Keys. Near-nightly downpours will soon fill bird baths and jet-ski tarps, bringing forth aegypti hatchlings from their dry-month dormancy. As these new generations take wing, Key West’s bloodmeal roulette will begin once again.