Coronavirus: The test of time
Hello Quartz readers,

Hello Quartz readers,
If there’s a running theme in your emails to us, it’s finding the positives in a tough moment. You’re taking all these homebound hours and filling them with ways of bettering yourselves. Keep at it. And keep ✉️ telling us about the journey.
Also, if you’re enjoying this newsletter—to the extent one can enjoy a pandemic newsletter—please ask others to sign up. We promise to treat them right.
Today, we want to talk about testing. Let’s get started.
Testing patience
When you think about a coronavirus test, you’re probably imagining a molecular one. That’s the test that starts with throat or nose swabs, where technicians look for the virus’s signature RNA molecules. But you may have also heard about “serologic tests,” sometimes marketed as a faster alternative. These tests search blood serum for signs of an immune response to the infection.
Serologic tests are faster. You don’t need a specialized machine to read the results, and the test doesn’t take a lot of extra supplies to process. Many such tests are in development by labs and private companies around the world.
But these tests aren’t always reliable when it comes to one pretty important question: whether you currently have the novel coronavirus.
Think about taking an STD test. The body takes time to figure out there’s an invader in its midst, and to start to fight back with antibodies. For HIV, that response could become detectable between three weeks and three months after exposure. For coronavirus, it seems to be at least eight days after the onset of symptoms. A serologic test won’t come back positive until that response has kicked in.
If you self-isolate long enough after an exposure and then get tested, you can be sure that a positive or negative is real—assuming the test works. But most of us can’t perfectly self-isolate (it’s not like just avoiding sex), and could be infectious for a week or more before an antibody response is detectable. These kinds of tests are prone to false positives, too, since other coronaviruses can provoke similar immune responses.
TLDR: Right now, serologic tests simply aren’t the best choice for diagnosis. But that doesn’t mean they’re not important. These tests could help identify healthcare providers and other essential workers who are now immune to the virus and could stop social distancing. Serologic tests can also help answer big-picture questions—How many people actually got infected? What portion of them died?—that are challenging to resolve with a limited number of molecular tests. And they can help us learn more about how long immunity lasts, which will make it easier to develop a vaccine.
Now, the challenge is to verify the accuracy of various new tests, and if they work, make a plan to deploy them—and hopefully allow scientists to track their results.
You asked
Does one become immune to Covid-19 after contracting and recovering from the disease?
Great question, Henry S. The answer is likely yes—but we don’t yet know how long that immunity lasts, or how strong it is.
Other coronaviruses, which can cause many versions of the common cold, tend to create immunity that lasts somewhere between one and three years. The coronavirus that caused the SARS epidemic in 2003—SARS-CoV, closely related to this pandemic’s SARS-CoV-2—induced longer-term immunity, around eight to 10 years. Regardless of how long immunity lasts, a second infection with a coronavirus will probably be milder than the first.

Are we there yet?
Early on, scientists and governments were slow to roll out testing, because they (fairly) wanted to make sure the tests were as accurate as possible. But those delays kept them from catching clusters of infections while they could still be contained. As a result, infections spread widely.
Today, everyone is agreed: We need much more testing to get a handle on Covid-19’s spread. But now there’s a backlog of diagnostic (i.e. molecular) tests. That’s because every single step of the molecular testing process demands a new set of highly specialized materials—many of which are in short supply. That creates not one but multiple potential bottlenecks. Here’s how it breaks down:
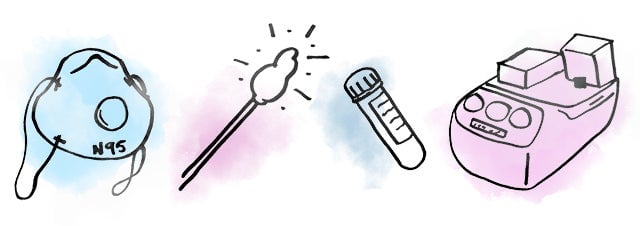
Sample collection: Healthcare workers wearing protective equipment use long, flexible swabs to get deep in the nose and throat, and ship the samples in special vials to labs.
RNA extraction: Lab techs use pre-assembled kits of chemicals (or as a scientist would say, reagents) to get viral RNA out of human cells.
Processing: Using another set of chemicals, lab techs convert the viral RNA to DNA, and introduce small sequences of DNA called primers, custom-designed to match up with parts of the coronavirus sequence.
PCR: Lab techs run the DNA through a machine that uses heating cycles to make copies of the coronavirus sequences, which usually takes four to six hours.
Coming up short
Since the beginning of the coronavirus outbreak, several groups have worked to keep tabs on the number of cases—across the globe, and country by country. But the same problem plagues every count: lack of tests.
- 🇦🇺 Australia: Lauded for its testing coverage (160,000 and counting), the country is now running into supply-chain issues.
- 🇮🇸 Iceland: Testing slowed after authorities realized there were only 2,000 testing swabs left in the country.
- 🇮🇳 India: Similar to the US, India’s desire to use tests from Indian companies is preventing testing at a critical stage of the outbreak
- 🇳🇬 Nigeria: As of March 22, only 152 people in the country had been tested.
- 🇷🇺 Russia: With few labs approved for testing, a pneumonia spike may be a sign of unreported cases.
Not everyone is falling behind, though:
- 🇸🇬 Singapore: A rapid swab test for symptomatic inbound travelers helped catch clusters before they could start.
- 🇰🇷 South Korea: More than 250,000 people have been tested in dozens of drive-through stations and nearly 600 clinics.
Delivering results
In the US, direct-to-consumer diagnostic tests have gained popularity over the past few years. When the FDA announced new, looser guidelines for testing on March 16, a crop of startups including Everlywell, Nurx, Forward, and Carbon Health saw their moment to offer at-home tests for Covid-19, approved by a telehealth doctor and shipped to your door. The problem is, the FDA hadn’t actually authorized them.
The tests would require customers to swab their own throats and noses—which, if done incorrectly, could lead to false negatives. After getting their wrists slapped by the FDA, some companies are still trying to make at-home tests work by bringing clinicians into homes to collect samples. Others are betting they can get the FDA to approve simpler blood-based tests for home use. But for now, no dice.
Essential reading
- The latest 🌏 figures: 585,040 confirmed cases; 129,812 classified as “recovered.”
- AI don’t know about that: Radlogics claims its AI can identify coronavirus in CT scans.
- Keeping watch: Could wearables be used to spot symptoms?
- Doing the most: Cuba’s coronavirus response is putting other countries to shame.
- Alexa MD: Voice assistants are helping humans work out their symptoms.
- Hmmm: Some Wuhan residents tested positive, then negative, then positive.
Our best wishes for a healthy day. Get in touch with us at [email protected], and live your best Quartz life by downloading our app and becoming a member. Today’s newsletter was brought to you by Tim McDonnell, Katie Palmer, Alexandra Ossola, Daniel Wolfe, Amrita Khalid, Susan Howson, and Kira Bindrim.