The UK Parliament just approved a controversial law to allow the creation of babies from the genes of three people. If clinical trials prove successful, the treatment—what many call “three-parent” IVF—will enable women with certain devastating genetic diseases to have their own biological children without risk of passing on their conditions.
Though the actual number of British women this will affect is small—nearly 2,500, according to recent estimates—the decision has potentially greater significance beyond British borders. For one thing, the procedure’s proponents in the US hope it will encourage the Food and Drug Administration to look more favorably upon the technology.
By cracking open the door to legally sanctioned genetic engineering, the decision’s significance also goes beyond the narrow benefits of the procedure, says Jamie Metzl, a senior fellow for technology and security at the Atlantic Council.
“The vote is a very, very big deal,” says Metzl, who has testified before the US Congress on genetic engineering run amok, an issue he explored in his 2014 novel Genesis Code. ”It’s the first time ever that a state [has] authorized human genetic engineering with a vote from parliament.”
“Three-parent” IVF and the genetic engineering revolution
Advocates of the three-parent treatment, otherwise known as mitochondrial donation (or as it’s called in the US, mitochondrial replacement therapy), draw a line between it and the DNA-tweaking procedures often called “gene therapy”—the sort of thing scientists in China are currently doing to monkeys.
The technology, they argue, doesn’t actually tinker with an embryo’s genes themselves, but with the cells parts that carry those genes. Specifically, it replaces an embryo’s mitochondrial DNA—the genetic information that tell the cells in our bodies how to develop—with that of another woman’s egg.
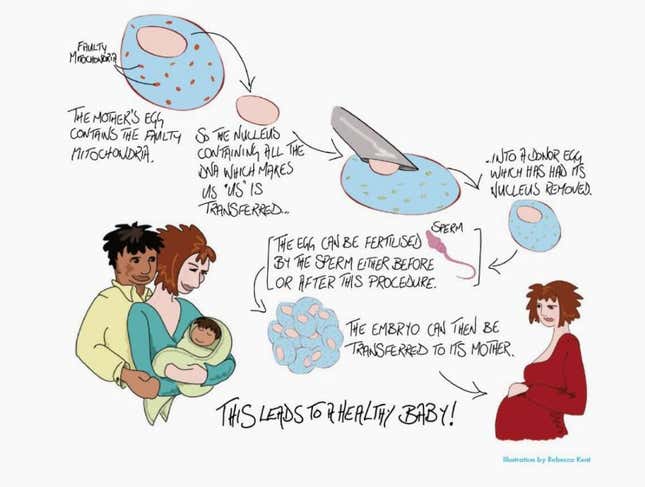
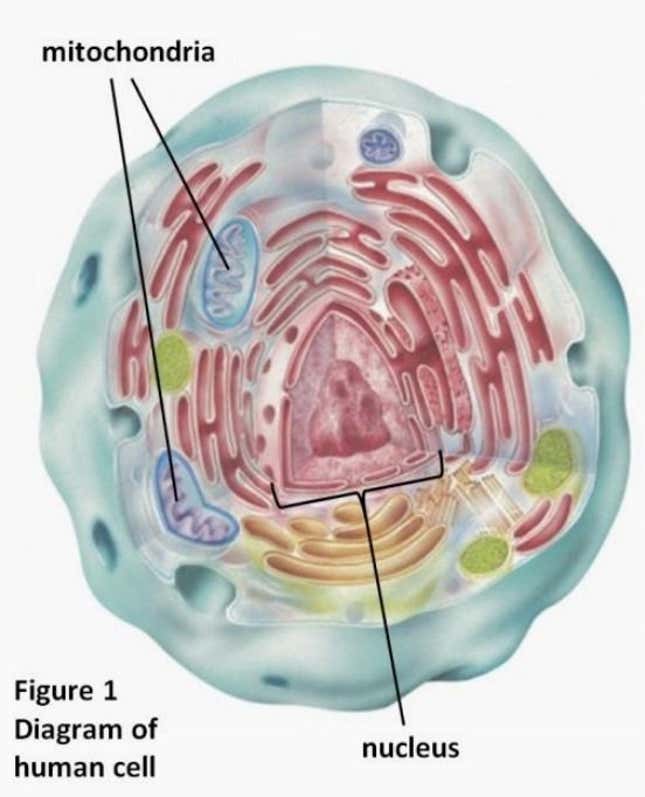
The mitochondria drift in a cytoplasmic goop surrounding the cell’s nucleus, the way egg whites surround a yolk. Some 99.9% of the embryo’s DNA is stored within that nucleus, which the baby will inherit from its biological parents. The just-approved procedure will replace the mother’s mitochondria with that from another woman’s egg.
Though they dictate only 0.1% of the baby’s genes, mitochondrial DNA—or “mtDNA” are unusually important: On a cellular level, they’re responsible for creating nine-tenths of our body’s energy. Mutations of mtDNA cause a litany of grave diseases, while problems with mitochondrial function are linked with degenerative disorders like diabetes, Parkinson’s and Huntington disease, and perhaps including severe autism.
There is no treatment for most of these. And though as many as one in 4,000 people develop severe mitochondrial diseases, the rate of mitochondrial mutation is much higher—as many as one in every 200 people are born with it.
This discrepancy has to do with the way mtDNA are passed on—a phenomenon responsible for the uniquely and tragically unpredictable transmission of mitochondrial disease.
Why would women need “three-parent” IVF?
Unlike the egg’s nucleus, which inherits both parents’s genes, the egg’s mtDNA are passed exclusively from mother to baby. (It’s that generational mtDNA handover that ancestry-tracking companies like 23andMe use to trace a person’s lineage.)
These mtDNA are scattered in each of the embryo’s cells at random. While a mother’s flawed mtDNA might make up only a small percentage of her overall mtDNA, giving her mild symptoms, by sheer rotten luck she could give her baby a higher concentration of these mutations, making the child grow up with a severe or even life-threatening version of the mother’s disorder. Some women don’t even realize their mtDNA are defective until after they’ve had children. This excellent New York Times Magazine article describes the experience of one such woman; though she is relatively healthy, her 22-year-old son is going blind and now has difficulty walking.
Before today, the best hope families had of avoiding this was to test the cells of early embryos to estimate the mutation concentration, implanting the one that appeared to have the fewest problems. But this procedure—called preimplantation genetic diagnosis (PGD)—is far from accurate, says Susan L. Solomon, CEO and co-founder of the New York Stem Cell Foundation (NYSCF), a non-profit that works on cures for major diseases using stem cell research, which the US government has banned itself from funding.
“Every time a couple where the woman carries this disease becomes pregnant, they have no idea until the child is born if their child is going to be sick,” says Solomon.
So women with mitochondrial disease who hope to have their own child must either adopt, carry another woman’s egg, or subject themselves to what Solomon and many geneticists call Russian roulette with their baby’s health.
Weighing the risks
Critics of this procedure point to testing on mice and flies that didn’t turn out well—a possible sign that the technology scrambles important communication between the mitochondrial and the nucleus. However, those results aren’t necessarily that instructive. Both creatures used in the testing are already heavily inbred, according to Columbia University’s Dr. Robert Klitzman and Dr. Mark Sauer, and University of Oxford’s Mark Toynbee, in a recent article (paywall). Meanwhile, other studies in macaques showed no sign of problems. Although we can’t know how safe the procedure is for humans, of course, until human trials begin, we also can’t say that the risks would necessarily outweigh the benefits, they argue.
Other critics believe the procedure interferes with what would have been a valuable human life, regardless of illness. “[The procedure] is not about finding a cure,” writes Dr. Peter Saunders for LifeNews.com. “It is about preventing people with [mitochondrial disease] being born.”
Some go further, essentially defending the existential rights of the cell material that is cast off in the procedure. ”In reality, it is a macabre form of eugenic cloning, in which a human being with a medical condition is killed and his or her parts are used to create a new human being with an improved biological state,” writes Maureen L. Condic for the socially conservative think tank, the Witherspoon Institute.
Though these arguments didn’t sway the UK Parliament, they are influencing US policymakers; Jeff Fortenberry, US representative from Nebraska, echoed these fears verbatim in an FDA budget meeting in March 2014.
“Clearly there are consequences here,” Fortenberry added, “and frankly these scenarios scare people and I would be very worried if they didn’t scare people.” Earlier in the hearing, former Iowa representative Tom Latham asked whether the government should be required to label three-parent children as genetically modified. While the FDA hasn’t yet ruled on US mitochondrial replacement trials, NYSCF’s Solomon says that at the moment, the outlook for FDA approval doesn’t look good.
American opposition
Greater resistance in the US may reflect the lack of government-led education on the issue compared to the UK, says David McKeon, chief of staff at NYSCF. Even though America’s homegrown mitochondrial replacement technology has been ready for human trials for nearly as long as the UK’s, there’s been little if any meaningful public education on the issue in the US.
“We’re sort of in a catch-up mode here,” says McKeon, who sees today’s parliamentary vote as a hopeful development. “We hope that in the US we can look at [the UK’s] example and that [can be a] guide in our country as well.”
US resistance to mitochondrial transfer might end up making things hard on the 12,000 American women of child-bearing age who suffer from mitochondrial disease, but there are larger issues at stake, says the Atlantic Council’s Metzl. There will likely be backlash if the FDA approves mitochondrial transfer in the absence of a rigorous public discussion about more ambitious genetic procedures that might follow.
“The big challenge is that the science is outpacing both our imagination and the regulatory framework,” says Metzl. “We need to have a much more significant conversation—not just about mitochondrial transfer, but the ongoing genetic revolution, because people don’t appreciate what we’re capable of doing now.”