This morning, the US Food and Drug Administration announced a new policy on trans fat, essentially banning it from processed foods.
Under the new rule, partially hydrogenated oils (PHOs), the biggest source of trans fat, are no longer “Generally Recognized as Safe” (GRAS), and cannot be added to foods after June 18, 2018, without specific approval from the FDA. In use since the 1950s, PHOs are often added to processed foods to extend their shelf life. They are frequently found in products like frosting, microwave popcorn, and coffee creamers.
“In this case, it has become clear that what’s good for extending shelf-life is not equally good for extending human life,” said Susan Mayne, director of the FDA’s Center for Food Safety and Applied Nutrition, in the announcement. The FDA said it expects the action to result in reductions coronary heart disease and fatal heart attacks.
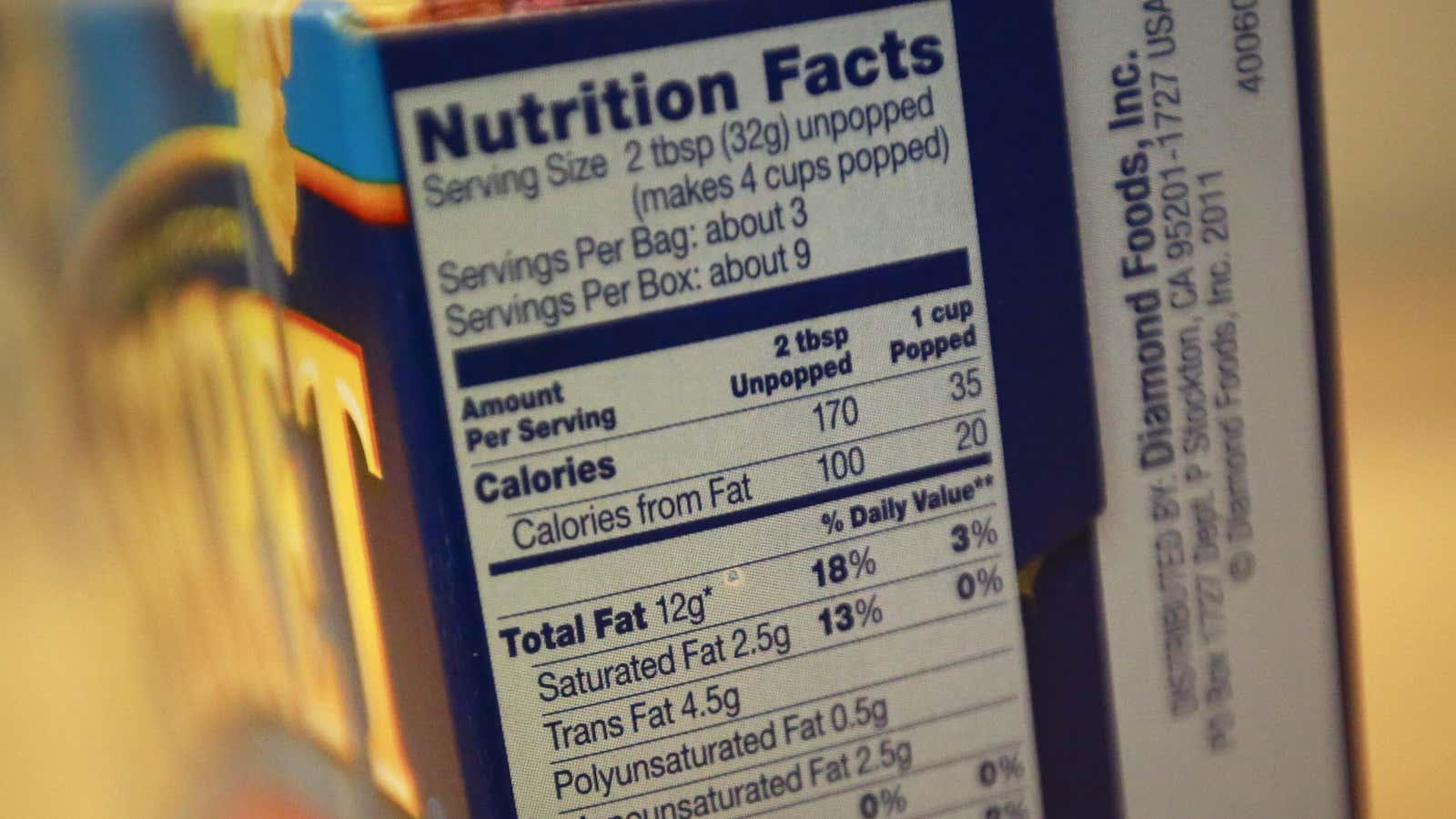
Since 2006, the FDA has required food manufacturers to disclose the amount of trans fat on the Nutrition Facts label of a product—but only if the product had 1/2 gram or more of trans fat per serving. A product with up to 1/2 gram of trans fat per serving could still declare “0g of trans fat” on its label, leaving consumers in the dark about PHOs unless they read the ingredients list.
Other sources of trans fat, such as those that occur naturally in some meat and dairy products, as well other processed trans fat that contain fatty acids that are chemically and structurally different than those in PHOs, do not fall under the purview of the new rule.
Although the two most common PHOs—partially hydrogenated soybean and cottonseed oils—were not officially listed as GRAS in the FDA’s regulations, the food industry has considered them and other PHOs to be GRAS based on “a history of use prior to 1958,” the FDA states in its final determination published in the Federal Register. Even though PHOs have been widely used, the history of use argument “is not sufficient to support continued GRAS status if new evidence demonstrates that there is no longer a consensus than an ingredient is safe.”