India’s homegrown Covid-19 vaccine became more attractive to the government’s expert committee in just one day.
Covaxin, jointly developed by pharmaceutical company Bharat Biotech and the Indian Council for Medical Research, was granted permission for “restricted emergency use” and “under clinical trial mode” by the drugs regulator’s subject expert committee (SEC) on Jan. 2. This approval was granted alongside the Oxford-AstraZeneca vaccine, to be manufactured by India’s Serum Institute.
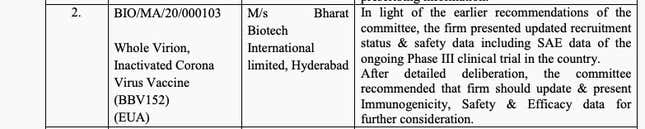
But just a day earlier, on Jan. 1, the SEC had noted that Bharat Biotech should “try to expedite the recruitment and may perform interim efficacy analysis for further consideration of restricted emergency use approval,” according to minutes of the meeting.
The SEC’s observations about Bharat Biotech’s Covaxin, which uses the Vero cell platform technology to transport the inactivated virus, seem to have changed over just three meetings. On Dec. 30, for instance, the SEC recommended that the firm “update & present Immunogenicity, Safety & Efficacy data for further consideration.” Covaxin is still in the process of conducting phase 3 human trials, and thus, does not yet have detailed efficacy data.
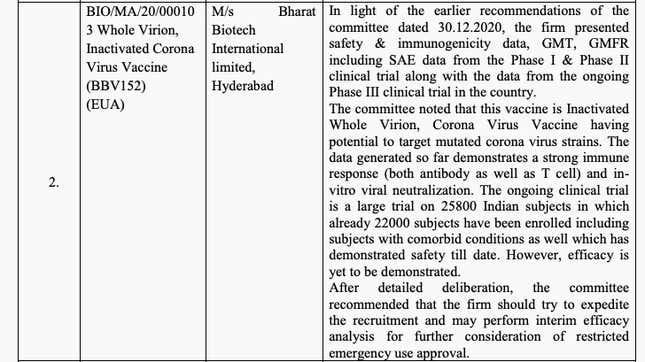
During the Jan. 1 meeting, the committee noted that Bharat Biotech had submitted safety and efficacy data from phases 1 and 2 of the trials. By then, it also believed, based on the company’s filings, that because Covaxin uses the “whole inactivated virion,” it would have the potential to target mutant coronavirus strains, the kind that has been detected in the UK.
This is a claim that Krishna Ella, chairman and managing director of Bharat Biotech, also made during a press conference on Jan. 4. He said he was fairly “confident” that the vaccine could be modified to protect against mutant strains, but that, as a scientist, he would need a week to confirm his hypothesis.
Despite this supposed potential, the committee’s minutes of the meeting from Jan. 1 noted that efficacy of the vaccine is yet to be determined.
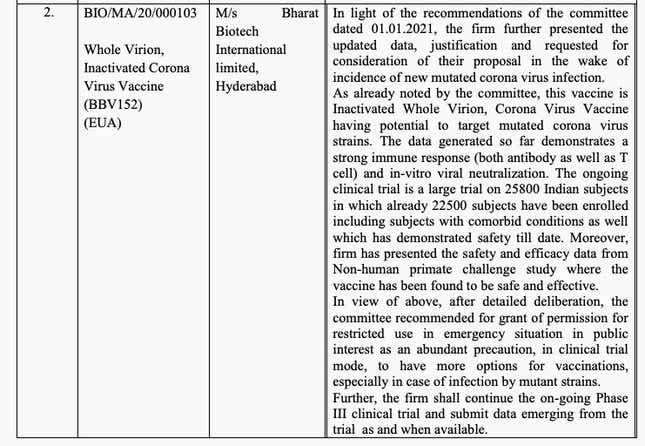
But on Jan. 2, it appears this potential gained more currency. Bharat Biotech’s data from non-human primate challenge studies found the vaccine to be safe and effective, the SEC noted. It also said, “The data generated so far demonstrates a strong immune response (both antibody as well as T cell) and in-vitro viral neutralization.” T cells are like the body’s memory centers for immune response. Lasting T cells are crucial for the longevity of a vaccine’s effectiveness.
“We are perplexed at the abrupt change in thinking of the SEC from the first two meetings to the third day on which the approval was recommended, while apparently discounting the need for efficacy data as the condition of the approval,” said Malini Aisola, co-convener of the All India Drug Action Network (AIDAN), an independent collective of healthcare nonprofits.
What truly tipped the scales in favor of an approval for Bharat Biotech seems to be its adaptability, as claimed by Ella, to fight mutant strains. In December, Pfizer’s vaccine partner BioNTech had said that the existing vaccine could fight various viral strains. But in the eventuality that a new vaccine is needed, “we could be able to provide a new vaccine technically within six weeks,” BioNTech’s co-founder Ugur Sahin told Agence France-Presse.
