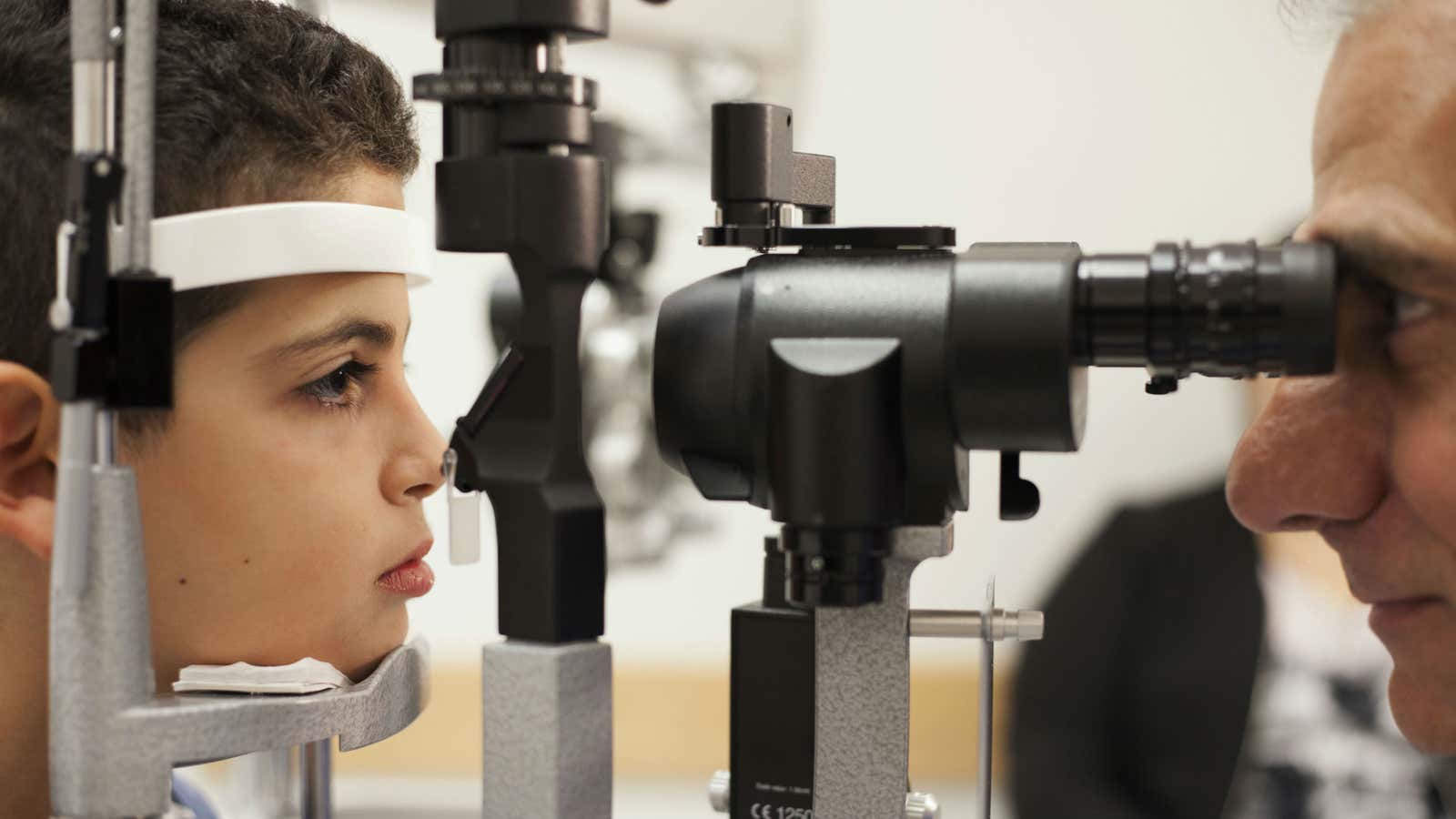
The US Food and Drug Administration just approved a new gene therapy that can treat an inherited disease.
On Tuesday Dec. 19, the FDA gave doctors the clearance to use Luxturna, a gene therapy, to treat the effects of biallelic RPE65 mutation-associated retinal dystrophy, a rare genetic eye disease. Patients have two copies of a mutated gene (one from each parent) called RPE65, which gradually leads to severely impaired sight in childhood and, in some cases, blindness by adolescence. Luxturna can stop and even in come cases reverse the effects of the condition.
Luxturna, made by Spark Therapeutics, is essentially a benign virus with a functional version of the gene. It’s injected directly into the retina. Once in the eye, the gene can do some of the work the patient’s own mutated copies cannot, which stops the progress of the disease, and in some cases, improves vision. The FDA approved Luxturna after a stage three clinical trial in which patients who received the drug could navigate an obstacle course at night significantly better than those who received a placebo injection. Some patients in the trial who got the injection recovered their ability to do things like read, play sports, and see the stars, according to NPR.
Spark Therapeutics hasn’t said how much they plan to charge for the treatment, although it’ll probably be expensive. Luxturna is only useful for a small population—there are about 1,000 to 2,000 people in the US with the condition it treats—but Spark is still expected to sell some $78 million worth of the drug in 2018, STAT reports. There’s a lot of uncertainty in these predictions because there is no set price for the drug, but analysts seem to think that those with the condition will be willing to pay high prices for the treatment. ”I think the price tag will be enormous—20 or 30 times the annual wages of the typical American,” Peter Bach, an epidemiologist at Memorial Sloan Kettering Cancer Center in New York, told NPR.
This is the third gene therapy the FDA has approved in the last five months. The first two were both cancer treatments. Earlier this year, two other experimental gene therapies (not yet FDA-approved) were used to effectively treat and reverse diseases caused by mutations.