In This Story
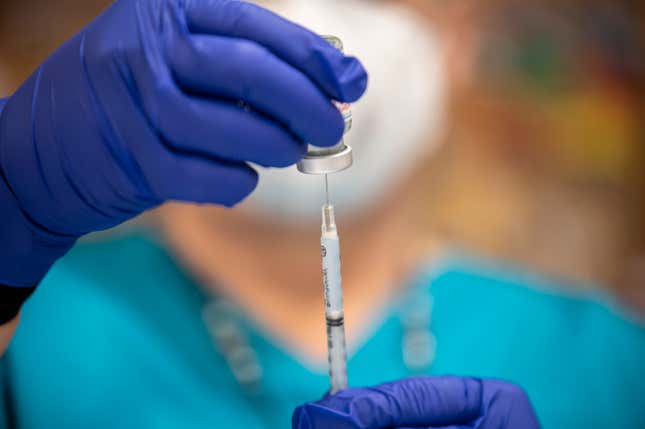
Noom will start offering smaller doses of compounded Wegovy (NVO+1.61%), the blockbuster weight-loss drug, as the digital health company continues to adapt to the growing demand for affordable weight-loss solutions and a changing market.
Reuters (TRI+0.10%) reported that Noom is rolling out personalized doses of its semaglutide drug to help patients manage their weight while minimizing side effects (such as nausea) that are a common concern with full doses of the drug.
Smaller doses can also help patients cut down on costs. Wegovy can cost upward of $349 for a 2.5-milligram vial. Meanwhile, Noom’s compounded semaglutide, offered through a telehealth model, allows patients to access the treatment at a fraction of the cost, starting at $149 for the first month.
Taking smaller doses of the medication has become popular due to the drugs’ expense and side effects, but Noom told Reuters that its goal isn’t to capitalize on the microdosing trend but rather to provide a more manageable and effective weight-loss solution, tailored to individual patient needs.
In a recent blog post, CEO Geoff Cook wrote that Noom’s GLP-1 RX Program with Smart Dose can address gaps “to provide a personalized, behaviorally integrated approach for patients who need it.”
Members who are prescribed a program with Noom’s SmartDose may start at a lower than commercially available dose and move their dosage up at a personalized pace based on individual response. Users will continue to adjust their dose through frequent check-ins to ensure the treatment is working best for them.
According to Reuters, Noom’s personalized approach could start with half of the standard 0.25-milligram starter dose of Wegovy and gradually increase to around half of the FDA-approved maximum dose of 2.4 milligrams over a 20-week period.
Noom has been offering the SmartDose program to patients clinically indicated since February.
The move comes as demand for weight-loss medications such as Wegovy has surged, driven by their ability to help patients potentially lose up to 20% of their body weight.
The FDA is moving to rein in unapproved compounded versions of semaglutide-based drugs such as Wegovy. These versions were temporarily permitted during shortages, but that exemption ends May 22. Noom, however, is betting its personalized prescribing model will still comply with FDA rules, which allow compounding for individual patients under specific conditions.
“There is a personalized — and there has always been a personalized — exception,” Cook, the company’s CEO, told Reuters.
Noom’s bet on smaller doses of its weight-loss drug comes as recent figures suggest the drug market may be seeing signs of slowing growth. Sales of Wegovy reached approximately 17.36 billion Danish kroner (about $2.64 billion) in Q1 2025, reflecting a 19% year-over-year increase — but also a 13% quarter-over-quarter decline, indicating potential signs of market stagnation. And the CEO of Ozempic maker Novo Nordisk just stepped down after the company’s stock dropped 50% in a year.
Competition has intensified, with Eli Lilly’s (LLY+0.74%) Zepbound and its coming GLP-1 pill adding pressure to the market for weight-loss drugs. Alongside these contenders, compounded alternatives to semaglutide are flooding the market, further complicating the landscape.